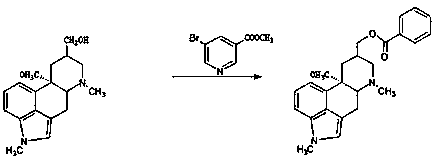
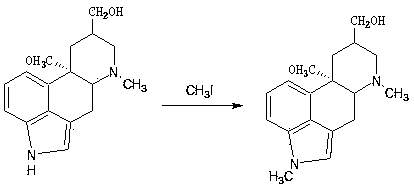
Synthetic method of nicergoline
A technology of nicergoline and a synthetic method, applied in directions such as organic chemistry, to achieve the effects of high yield, good quality, and simplified post-treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
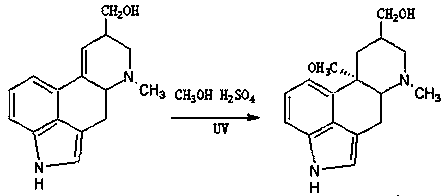
[0027] (1) Photocatalytic addition reaction to form 10-methoxy-dihydroergot alcohol
[0028] Using ergot alcohol as the starting material, the addition of methanol and ethylenic bonds under strong acidic conditions is carried out by ultraviolet light catalysis, and the 9, 10 double bonds are added to form 10-methoxy-dihydroergo alcohol.
[0029] Specifically, according to the mass ratio of ergool:methanol:concentrated sulfuric acid of 1:4:1.8, 5kg of ergool was added to a 50-liter photocatalytic reaction kettle, and then 20kg of methanol was added, and the ergool was dissolved and clarified by stirring. Then dropwise add 9kg of concentrated sulfuric acid with a mass concentration of 98%, close the reaction kettle, turn on the ultraviolet lamp of 6kW power, keep the reaction temperature at 25~30°C, after reacting for 4 hours, take a sample, and detect the reaction through TLC, so that the raw material spot is less than The control spot with a concentration of 5% in the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com