Cluster-base crystalline material, and preparation method and application thereof
A technology for base crystals and adsorption materials, applied in chemical instruments and methods, other chemical processes, etc., can solve the problems of thermal stability frame disadvantage, limited application, frame shrinkage, etc., and achieve easy mass preparation, mild conditions, and good resistance. The effect of high temperature performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of cluster-based crystalline material
[0032] Mix 30mg of cobalt chloride hexahydrate and 6mg of isonicotinic acid ligand in a beaker to obtain a mixture, then add 2mL of DMA, stir at room temperature for 30min to obtain a mixture; transfer the above mixture into a 10mL vial with a screw stopper, add 0.5 mL tetrafluoroboric acid aqueous solution, baked at 120°C for 36 hours, filtered and separated to obtain the product after cooling down to room temperature; washed the above solid with DMA for 3 times to obtain red strip-shaped hybrid material crystals, based on organic ligands The calculated yield is 68%. The main infrared absorption peaks are: 3388m, 3116w, 1615s, 1558s, 1401s, 1317m, 1185s, 1077s, 835m, 775s, 632s.
Embodiment 2
[0033] Embodiment 2: Preparation of cluster-based crystalline material
[0034] Mix 28mg of cobalt chloride hexahydrate and 8mg of isonicotinic acid ligand in a beaker to obtain a mixture, then add 5mL of DMA, stir at room temperature for 30min to obtain a mixture; transfer the above mixture into a 10mL vial with a screw stopper, add 1.5 mL tetrafluoroboric acid aqueous solution, baked at 120°C for 36 hours, filtered and separated to obtain the product after cooling down to room temperature; washed the above solid with DMA for 3 times to obtain red strip-shaped hybrid material crystals, based on organic ligands The calculated yield is 65%. The main infrared absorption peaks are: 3388m, 3116w, 1615s, 1558s, 1401s, 1317m, 1185s, 1077s, 835m, 775s, 632s.
Embodiment 3
[0035] Embodiment 3: the preparation of cluster-based crystalline material
[0036] Mix 31mg of cobalt chloride hexahydrate and 5mg of isonicotinic acid ligand in a beaker to obtain a mixture, then add 2mL of DMA, stir at room temperature for 30min to obtain a mixture; transfer the above mixture into a 10mL vial with a screw stopper, add 3mL The aqueous solution of tetrafluoroboric acid was baked at 120°C for 36 hours, then filtered and separated to obtain the product after cooling down to room temperature; the above solid was washed 3 times with DMA to obtain a red striped hybrid material crystal, calculated based on organic ligands The yield was 60%. The main infrared absorption peaks are: 3388m, 3116w, 1615s, 1558s, 1401s, 1317m, 1185s, 1077s, 835m, 775s, 632s.
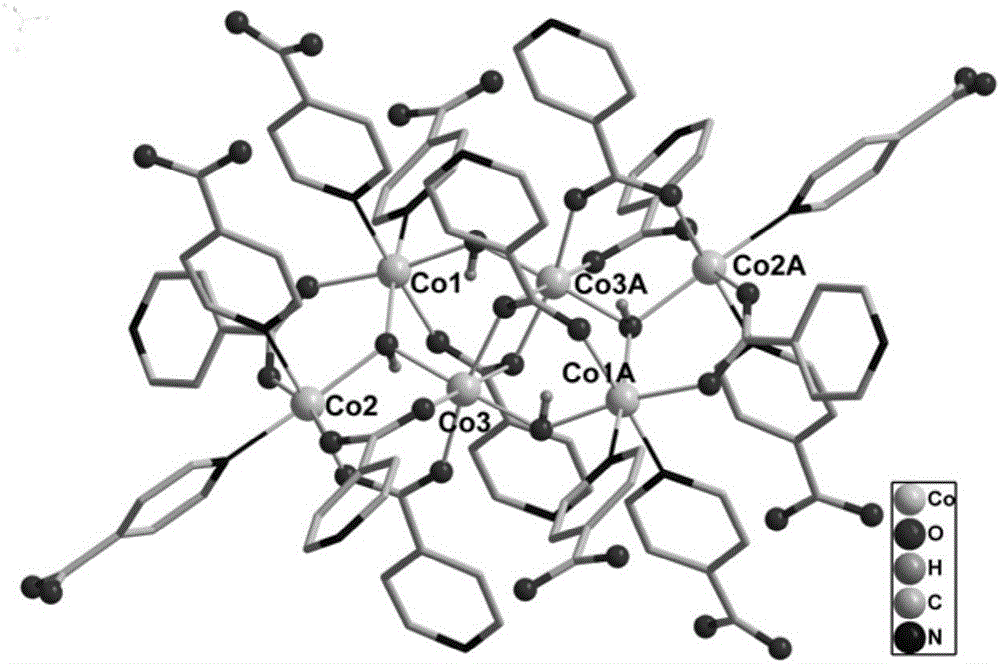
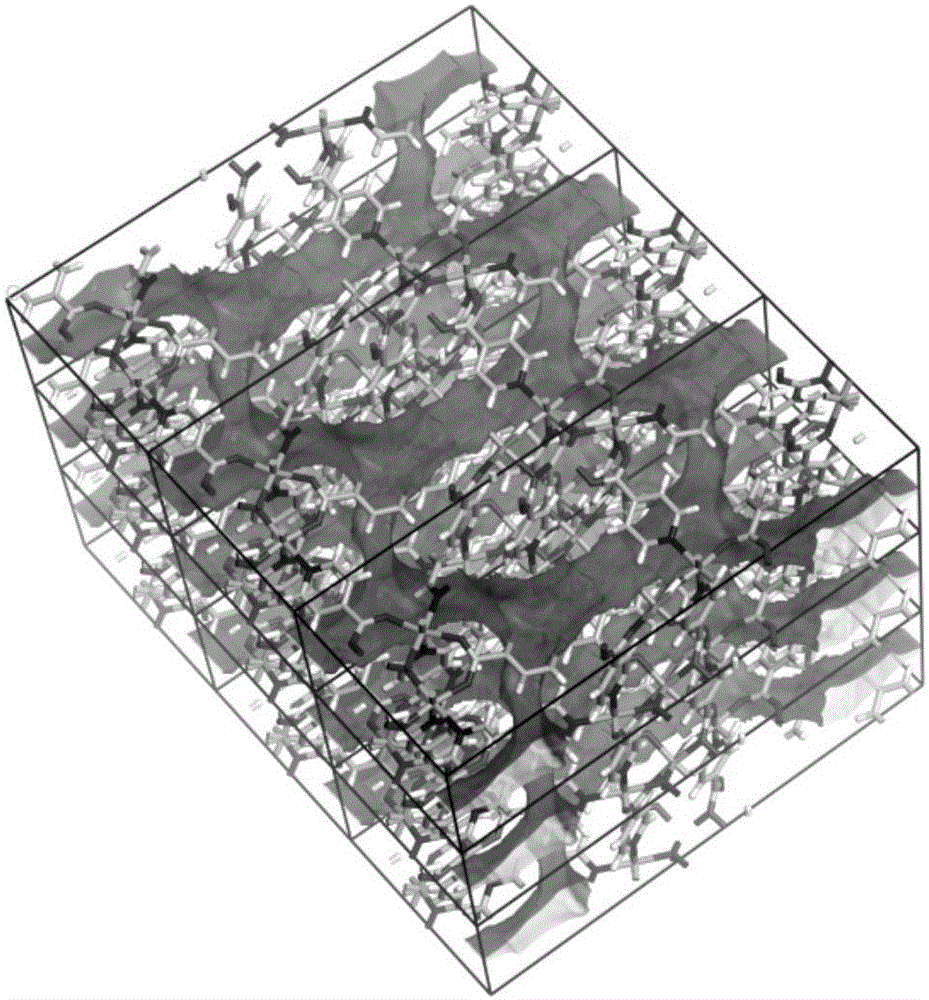
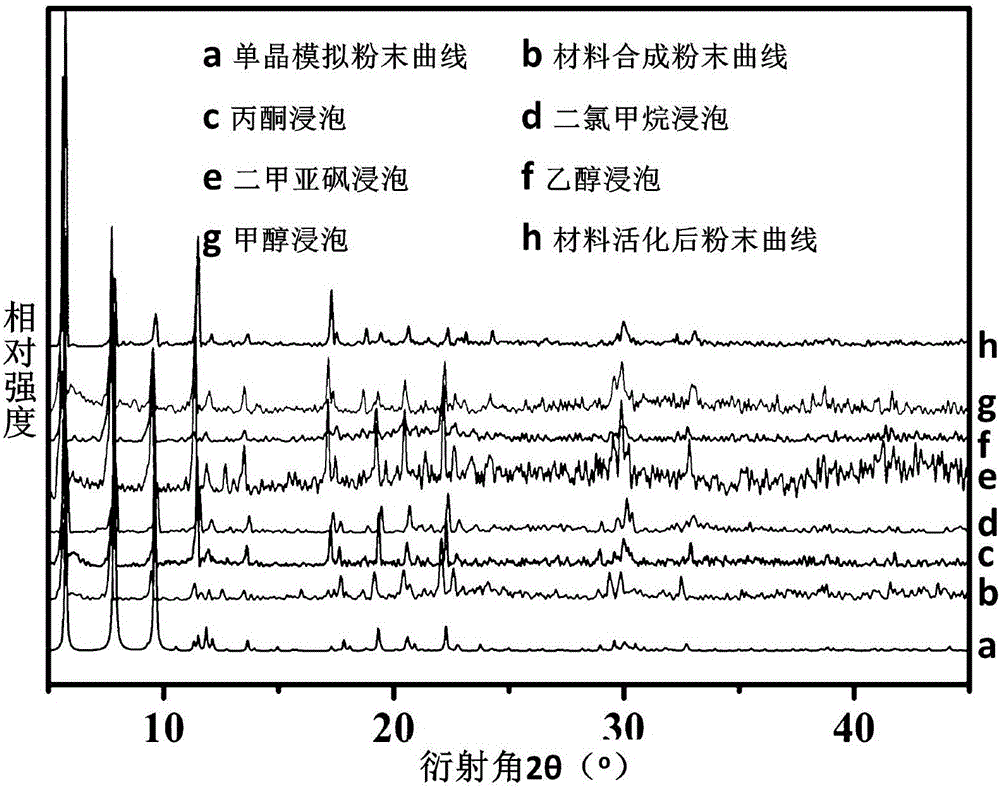
[0037] Get the further characterization of the obtained carboxy-based crystalline material in Example 1, and its process is as follows:
[0038] (1) Determination of crystal structure
[0039] Under a polarizing...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com