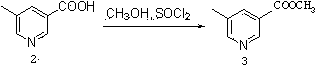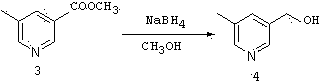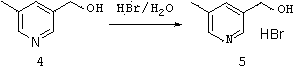Method for preparing 5-methyl-3-bromomethylpyridine hydrobromide
A technology of bromomethylpyridine hydrobromide and bromomethylpyridine bromide, applied in the preparation of 5-methyl-3-bromomethylpyridine hydrobromide, pharmaceutical intermediate 5-methyl-3- In the field of bromomethylpyridine hydrobromide, it can solve the problems of complex preparation process and low yield, and achieve the effects of simple post-treatment, simple reaction, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] compound ( 3 )Synthesis:
[0039]
[0040] In a 1000mL four-necked bottle, add the compound ( 2 ) 5-methylnicotinic acid (100.0g, 0.73mol) and 500mL of methanol, under the protection of nitrogen, add thionyl chloride (110mL, 1.5mol) dropwise, and keep the temperature at 20-25°C. After the dropwise addition, heat to reflux for 4 hours, remove methanol by evaporating under reduced pressure, add 200mL of ice water, neutralize to weakly alkaline (pH 7~10) with saturated sodium carbonate solution, extract with ethyl acetate, and use saturated brine for the organic phase After washing, drying over anhydrous sodium sulfate, the solvent was evaporated under reduced pressure to obtain a white solid (108.2 g, 98.2%).
[0041] Melting Point: 44.9-45.4℃. MS(ESI, M+H) + : 151.95. 1 H NMR (400Hz, CDCl 3 ) δ 9.03(1H, s), 8.60(1H, s), 8.11(1H, s), 3.95(3H, s), 2.40(3H, s). IR(KBr, cm -1 for C 8 h 9 NO 2 : C: 63.56; H: 6.00; N: 9.27. Found: C:63.57; H: 6.01; N: 9,30.
[004...
Embodiment 2
[0054] compound ( 3 )Synthesis:
[0055]
[0056] In a 1000mL four-necked bottle, add the compound ( 2 ) 5-methylnicotinic acid (100.0g, 0.73mol) and 500mL of methanol, under the protection of nitrogen, add thionyl chloride (80mL, 1.1mol) dropwise, and keep the temperature at 20-25°C. After the dropwise addition, heat to reflux for 4 hours, TLC shows that the reaction is not complete, add 30 mL of thionyl chloride, continue to heat and reflux for 4 hours, and the reaction is complete. Evaporate under reduced pressure to remove methanol, add 200 mL of ice water, neutralize to weak alkalinity with saturated sodium carbonate solution, extract with ethyl acetate, wash the organic phase with saturated brine, dry over anhydrous sodium sulfate, and remove the solvent by evaporation under reduced pressure to obtain White solid (104.6g, 94.8%).
[0057] compound ( 4 )Synthesis:
[0058]
[0059] In a 100mL three-neck flask, add the product from the previous step ( 3) methy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com