Synthesis method of (R, S-) nicotine
A synthetic method, nicotine technology, applied in chemical instruments and methods, organic chemistry, physical/chemical process catalysts, etc., can solve the problems of long reaction cycle, many steps, low yield of process route, etc., and achieve high synthesis specificity , mild reaction conditions and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
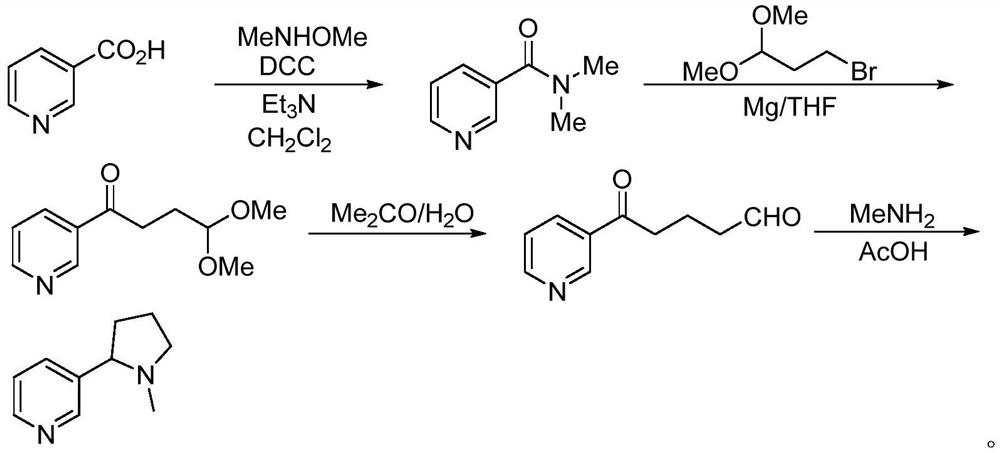
[0034] The synthetic method of described (R, S-) nicotine comprises the steps:
[0035] (1) In a reaction vessel, add methyl nicotinate and N-butenylpyrrolidone to prepare N-butenyl-3-benzoyl-1-pyrrolidone in a suitable solvent under alkaline conditions; after the reaction , adding a suitable aqueous solution of inorganic acid to carry out hydrolysis reaction; after cooling, adding lye to adjust the pH value to 10, extracting with a suitable organic solvent, separating the organic phase, concentrating, and distilling the mother liquor to obtain an enamine intermediate;
[0036] (2) Disperse the enamine intermediate in a suitable solvent, add a metal reduction catalyst under light, and perform a reduction reaction at a suitable temperature; perform a methylation reaction after the reaction, filter, concentrate the filtrate, and distill the bottom liquid The target product (R,S-)nicotine was obtained.
[0037] In step (1), the suitable solvent is one or more of diethyl ether, d...
Embodiment 1
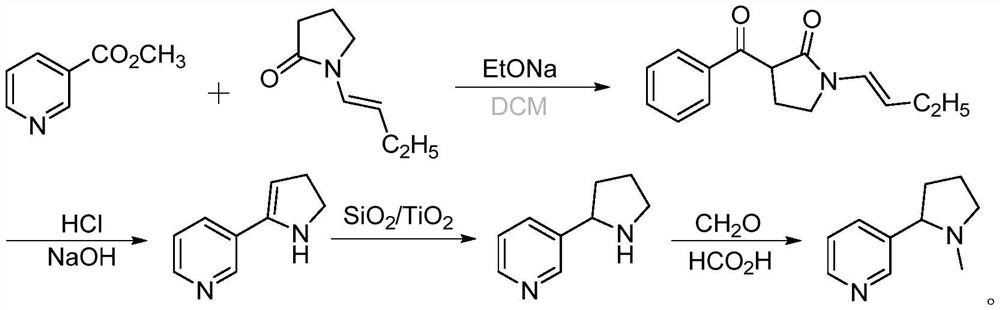
[0041] Add 150g of sodium ethoxide and 1000mL of dichloromethane into the three-necked flask, install the magnet and condenser, and replace the system with nitrogen; mix 100g of methyl nicotinate and 117g of N-butenylpyrrolidone with 200mL of dichloromethane, and the system Slowly raise the temperature to 50°C, add the mixed solution dropwise, continue to keep warm for 3 hours, then cool to 5°C, add 6N (ie: 6mol / L) phosphoric acid dropwise to quench the reaction, adjust the pH value to 4 and react for 8 hours; At the end of the reaction, 6N lye was added to adjust the pH value to 10, and dichloromethane was added to extract three times; the organic phases were combined, dried, concentrated, and distilled to obtain 9.158 g of a light yellow oily enamine intermediate, with a yield of 81%.
Embodiment 2
[0043] Disperse the obtained enamine intermediate in 100 mL of methanol and add 1 g of SiO 2 / TiO 2 Catalyst (SiO 2The mass fraction is 5.5%), placed in a reaction bottle, after nitrogen replacement, irradiated with light of 350nm wavelength, stirred at room temperature overnight; after the reaction, add formaldehyde solution to control the temperature at 30°C, add formic acid dropwise, and monitor the reaction by TLC. After the completion of filtration, the filtrate was concentrated to obtain crude (R,S-)nicotine, which was distilled to obtain 8.3g of colorless and transparent pure product (68-70°C, 0.2mmHg), with a GC purity of 99.6% and a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com