Composition containing cannabidiol and use of composition in treatment of systemic inflammatory response syndrome
A systemic inflammatory response and cannabidiol technology, applied in the field of medicine, can solve problems such as difficulty in obtaining ideal results and failure to find key nodes, and achieve the goal of reducing liver first-pass effect, reducing cytokine storm, and improving treatment level and curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation of embodiment 1 microemulsion preparation
[0067] (1) Preparation of emulsifier: Measure 0.5 ml of Tween 80, 0.5 ml of absolute ethanol and 9 ml of normal saline, and mix them with a magnetic stirrer to prepare 10 ml of drug dissolution and emulsifier, which is recorded as the vehicle group.
[0068] (2) Preparation of CBD microemulsion: Measure 0.5 ml of Tween 80 and 0.5 ml of absolute ethanol, add 40 mg of CBD crystal powder (CBD content>98%), mix with a magnetic stirrer for 2 minutes, heat at 60°C and blow , continue to vortex for 5 minutes to promote its full dissolution, mix with 9 ml of normal saline, continue to vortex for 30 minutes, and record it as the CBD microemulsion group.
[0069] (3) Preparation of GABA microemulsion: Measure 0.5 milliliters of Tween 80 and 0.5 milliliters of dehydrated ethanol, mix with a magnetic stirrer, then mix with 9 milliliters of normal saline containing 300 milligrams of GABA (purity 98%), and vortex for 30 Min...
Embodiment 2
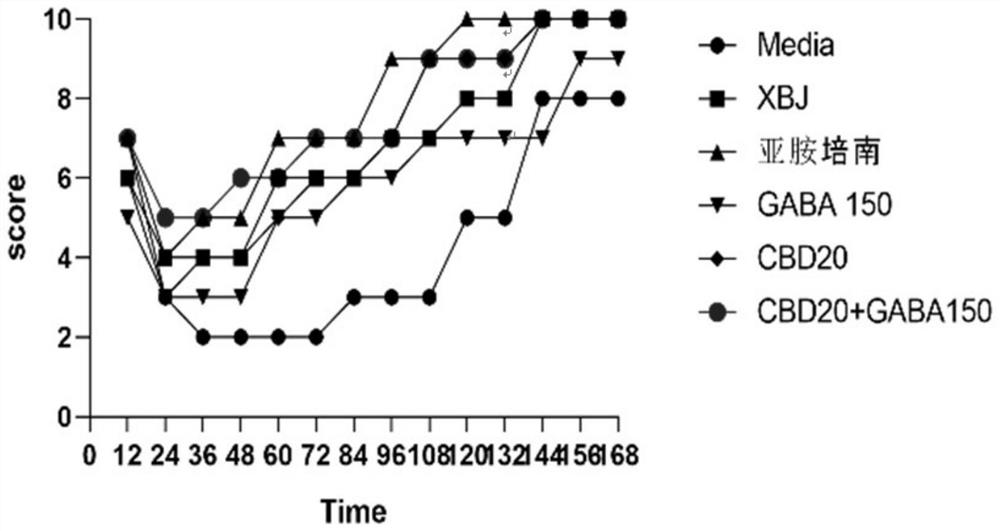
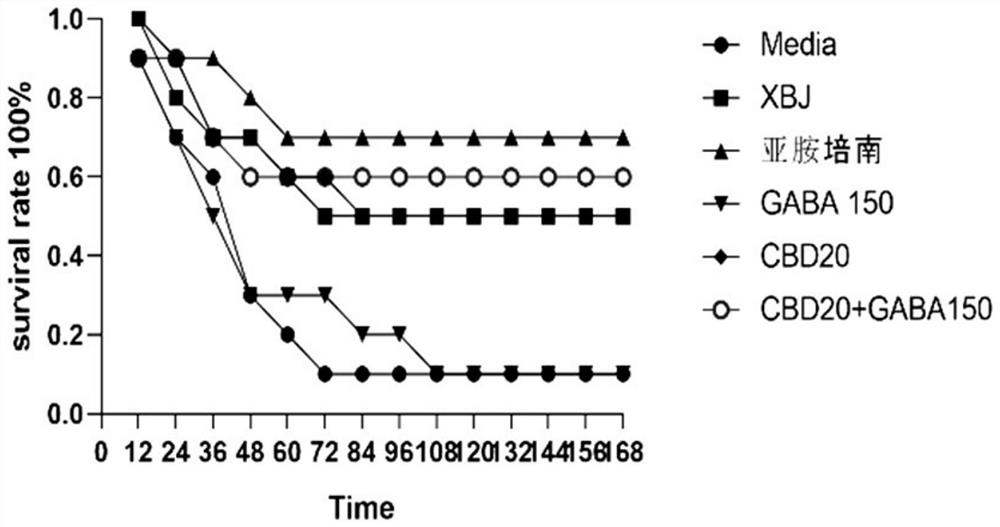
[0072] Example 2 The effect of CBD and combined intervention with GABA on the overall performance and survival rate of mice with sepsis (CLP)
[0073] A mouse model of sepsis was constructed by cecal ligation and puncture (CLP). Under the condition of no anti-infection (antibiotics) treatment, the overall survival of sepsis mice in different groups was observed for one week after the intervention of CBD and CBD combined with GABA preparations. status and survival.
[0074] 1) Animal model
[0075] Sixty C57 wild-type mice, male, weighing 20-22 grams, were used. Divided into 6 groups, 10 in each group. Routine 0.5% chloral hydrate intraperitoneal injection anesthesia, using cecal ligation and puncture (CLP) to establish a mouse model of sepsis.
[0076] 2) Model grouping
[0077] (1) CLP+vehicle group (Vehicle): after laparotomy, cecum ligation and perforation were performed, and 100 microliters of the prepared solvent (Vehicle) for dissolving drugs were injected intraperit...
Embodiment 3
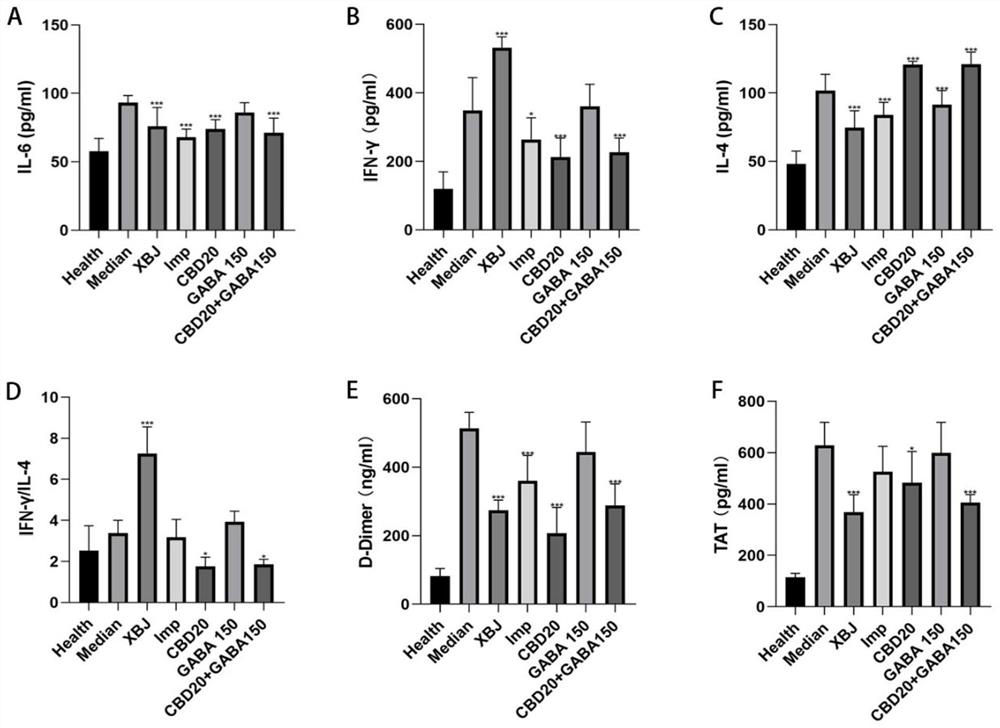
[0092] Example 3 Effect of CBD and combined intervention with GABA on peripheral blood cytokines and coagulation function in mice with sepsis (CLP)
[0093] 1. Animal model
[0094] C57 wild-type mice, male, weighing 20-22 grams, were routinely anesthetized by intraperitoneal injection of 0.5% chloral hydrate, and a mouse sepsis model was established by cecal ligation and puncture (CLP). Injury operation and treatment were the same as in Example 2, and a group of healthy controls was added.
[0095] 2. Treatment groups: 70 mice in total, 10 in each group
[0096] (1) Healthy control group: the mice were fasted for 12 hours before operation like other mice, without any surgical treatment, and were given food and water at the same time as other groups after operation.
[0097] (2)CLP+medium group
[0098] (3) CLP+CBD (20mg / kg)
[0099] (4) CLP+GABA (150mg / kg)
[0100] (5) CLP+CBD(20mg / kg)+GBAB(150mg / kg)
[0101] (6) CLP+ Taineng (imipenem cilastatin sodium for injection) (25...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com