Patents
Literature
67 results about "Virus processing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The main idea behind viral processing is to stop the viruses in a given sample from contaminating the desired product. The two most widely used methods of viral processing are viral removal and viral inactivation. The former is a method in which all viruses are simply removed from the sample completely. The latter method is one in which the viruses may remain in the final product, but in a non-infective form. These techniques are used widely in the food and blood plasma industries, as those products can be harmed by the presence of viral particles. Some of the more common viruses removed by these methods are the HIV-1 and HIV-2 viruses; hepatitis A, B, and C; and parvoviruses. The methods used in the plasma industry have been summarized (Horowitz B., Minor P., Morgenthaler J. J., Burnouf T., McIntosh R., Padilla A., Thorpe R. and van Aken W. G. Who Expert Committee on Biological Standardization. World Health Organ Tech Rep Ser. 924: 1-232, 2004.) In some cases, however, it is the virus itself that is the desired product, as is often the case with the HIV. In many cases, researchers may be trying to extract the viruses from the blood for study, not specifically for blood purification. It is also common to use these types of techniques to remove particles produced as a result of viral infection.
Methods, software and apparatus for detecting and neutralizing viruses from computer systems and networks
InactiveUS20080263670A1Minimizing chanceMitigating unwanted response time-outMemory loss protectionError detection/correctionData streamNetwork packet
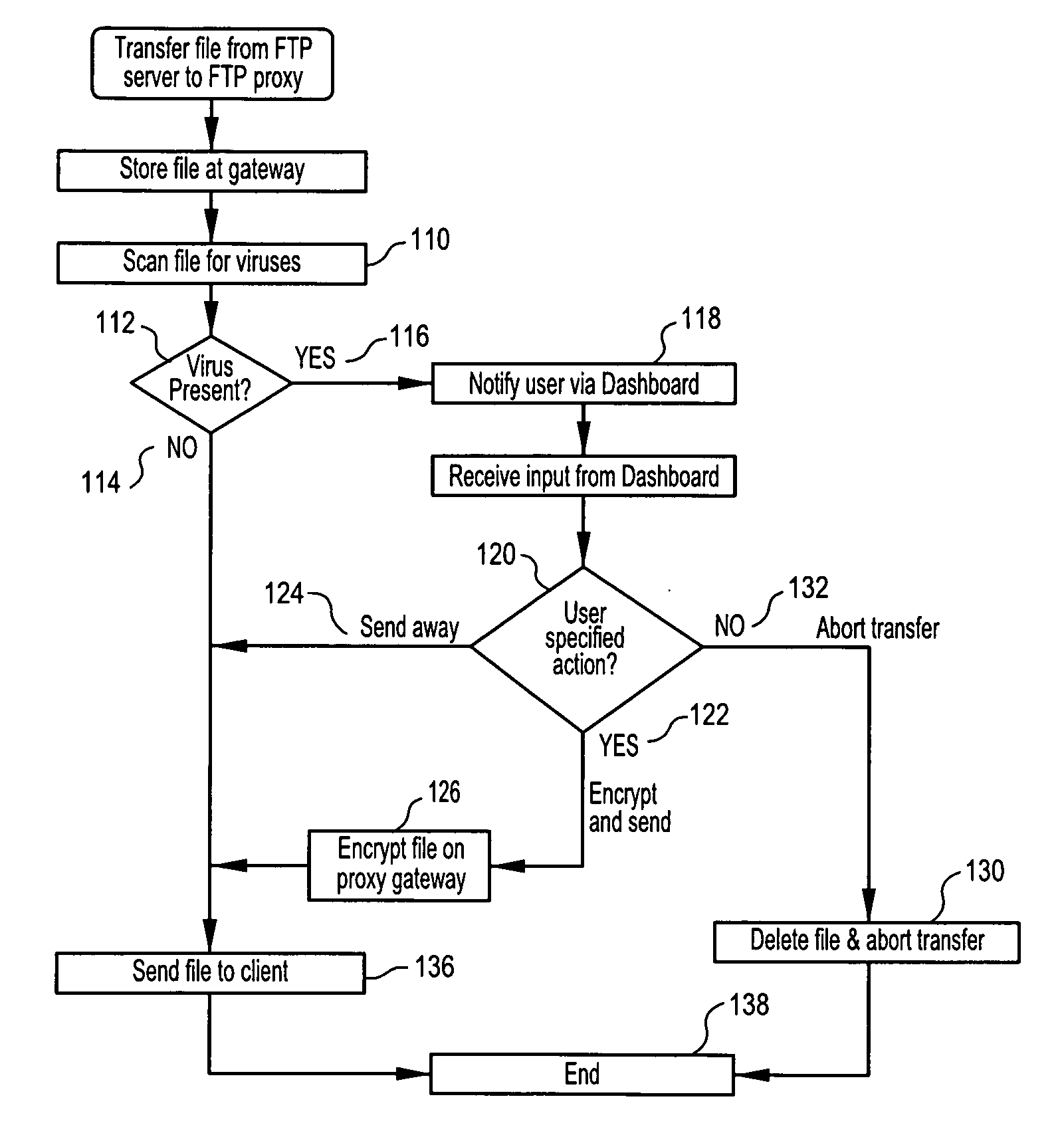
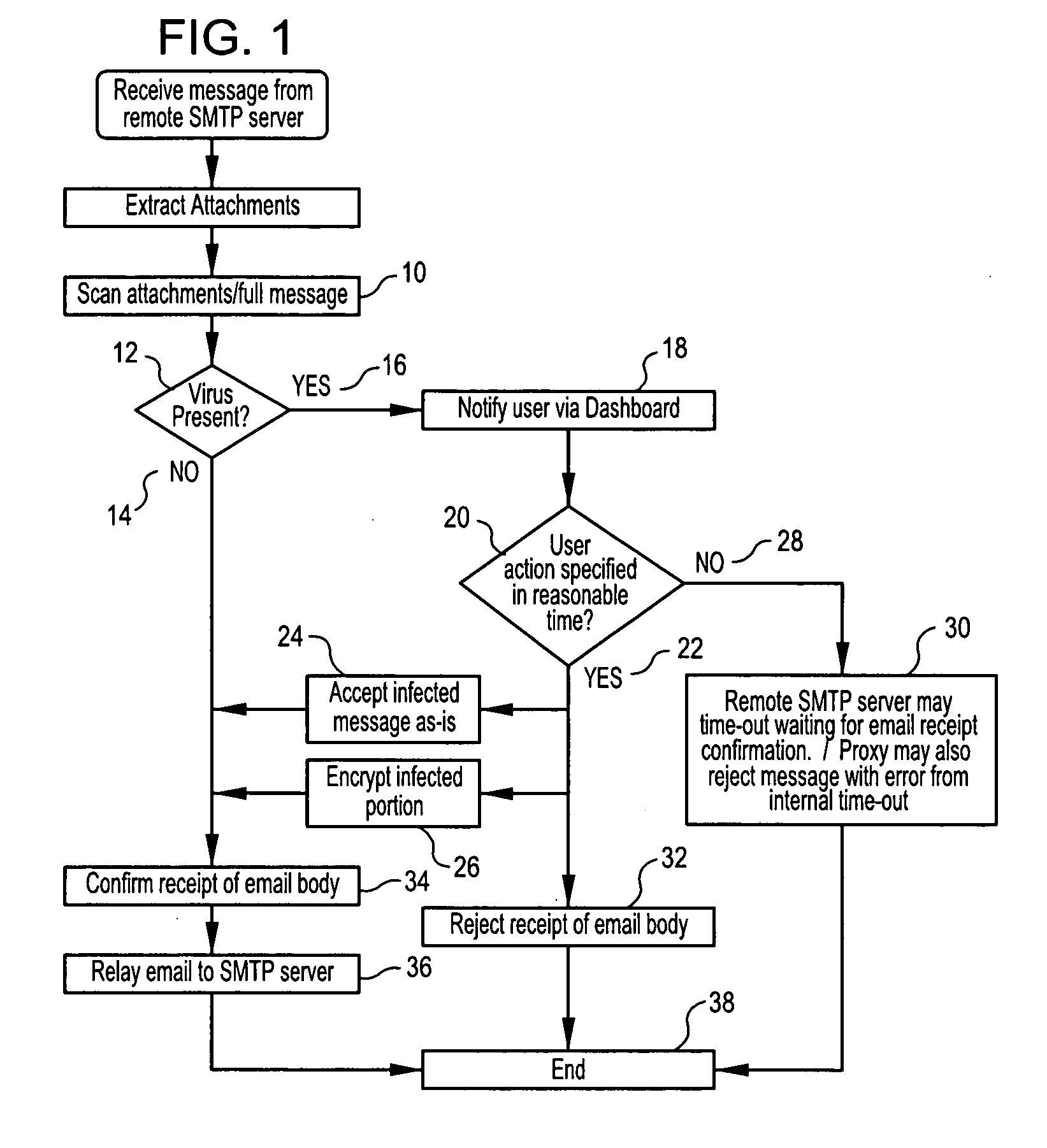
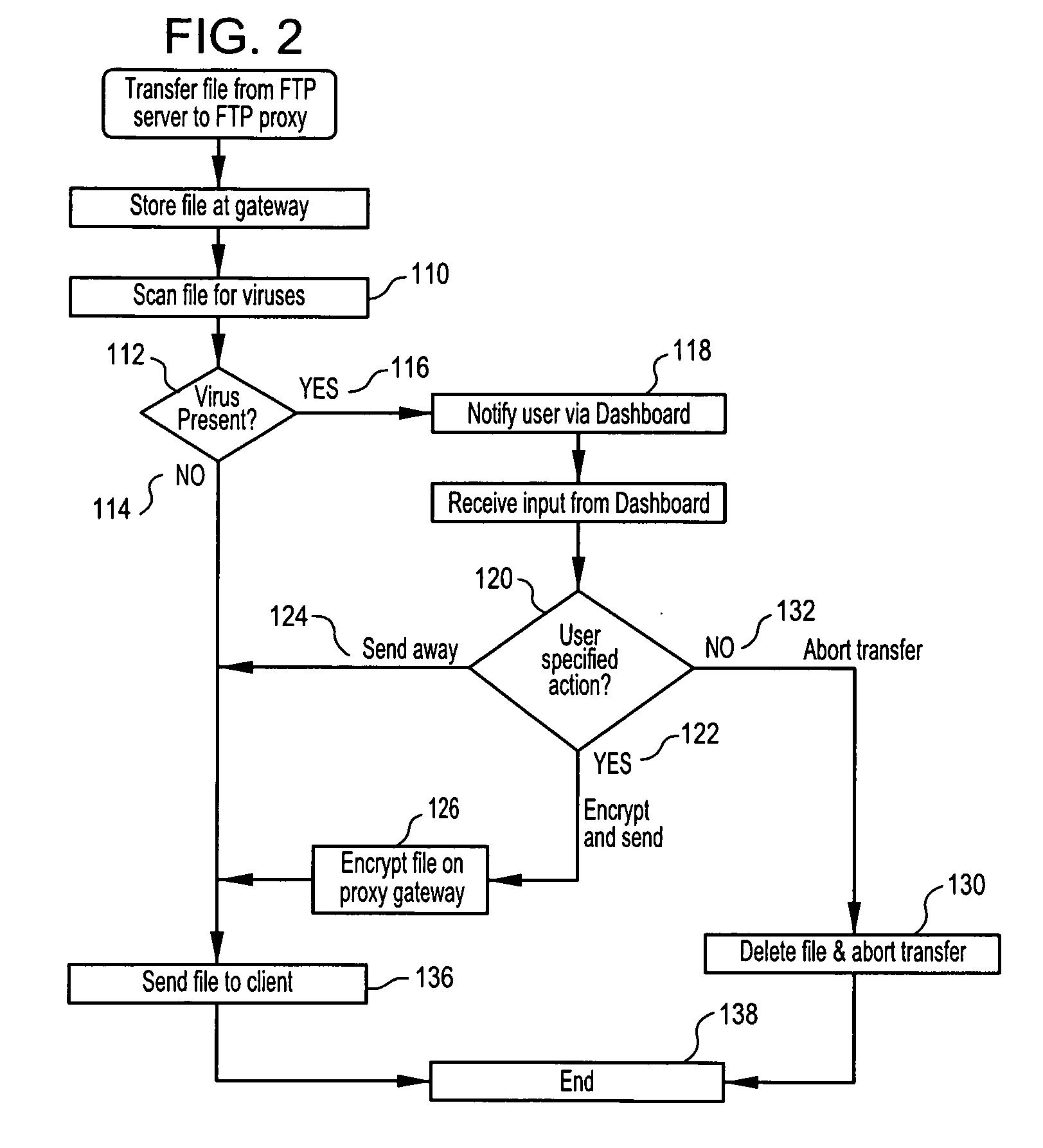
Methods, software or computer programs, and apparatus for detecting viruses and mitigating their harm to computers communicating through a gateway node to another network are disclosed. Upon detection of a virus in an incoming data stream or plurality of data packets directed to a gateway device or node, the data requesting recipient is notified and provided with a plurality of pre-defined virus handling action options. If the recipient, or designated proxy, fails to select an action option, then a random selection is made. If a selection is made, then that selection, to the exclusion of other action options, is carried out. Thus, the recipient is empowered to dynamically select, as circumstances dictate and without future prejudice, the appropriate response upon detection of a particular virus. Action options may include data encryption and forwarding with recipient notification, or where email is the vector, attachment removal and location link insertion may be used. Software embodiments of the invention provide the machine readable instructions to carry out the methods according to the invention.
Owner:WIRESOFT OPERATING COMPANY
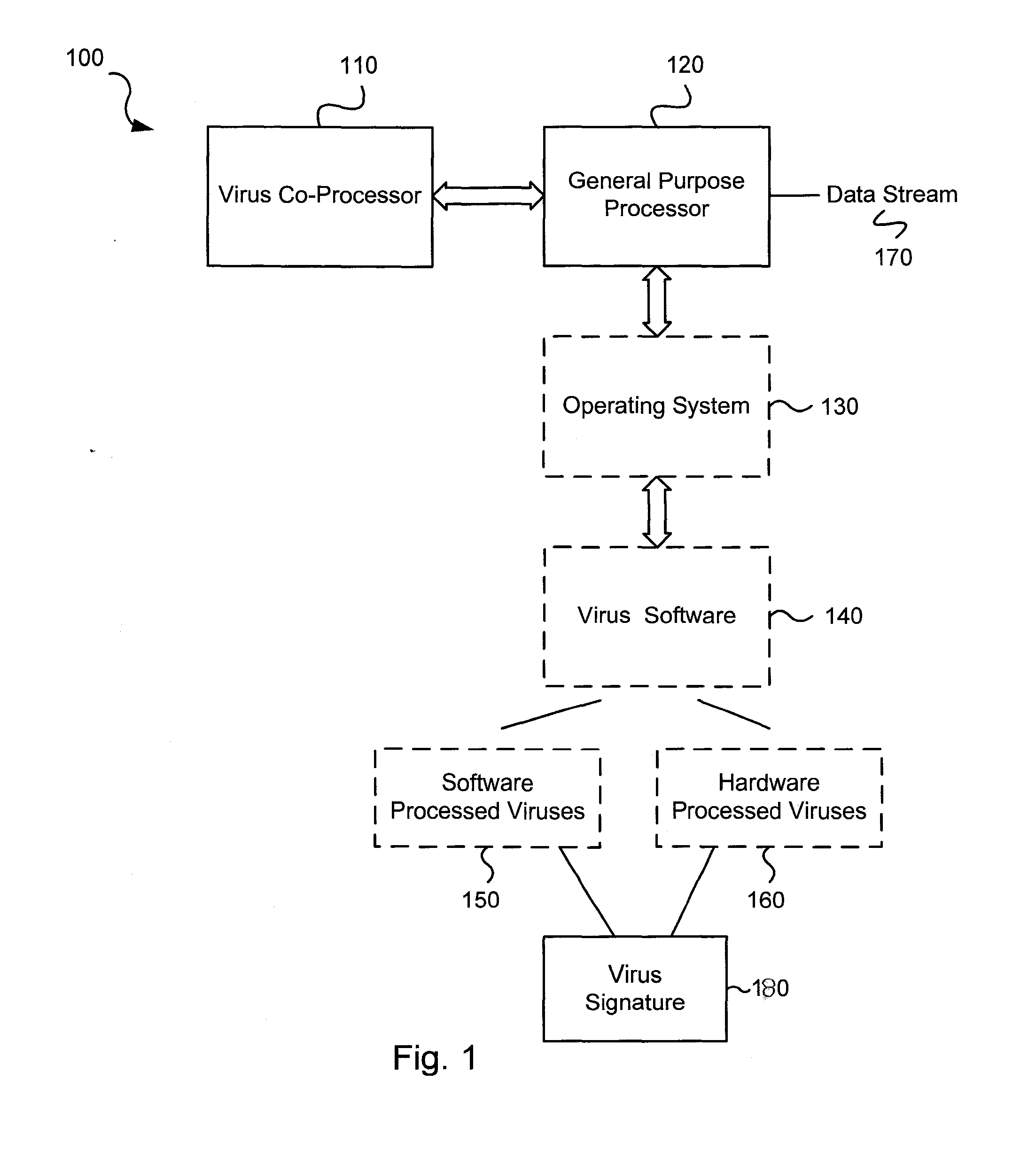
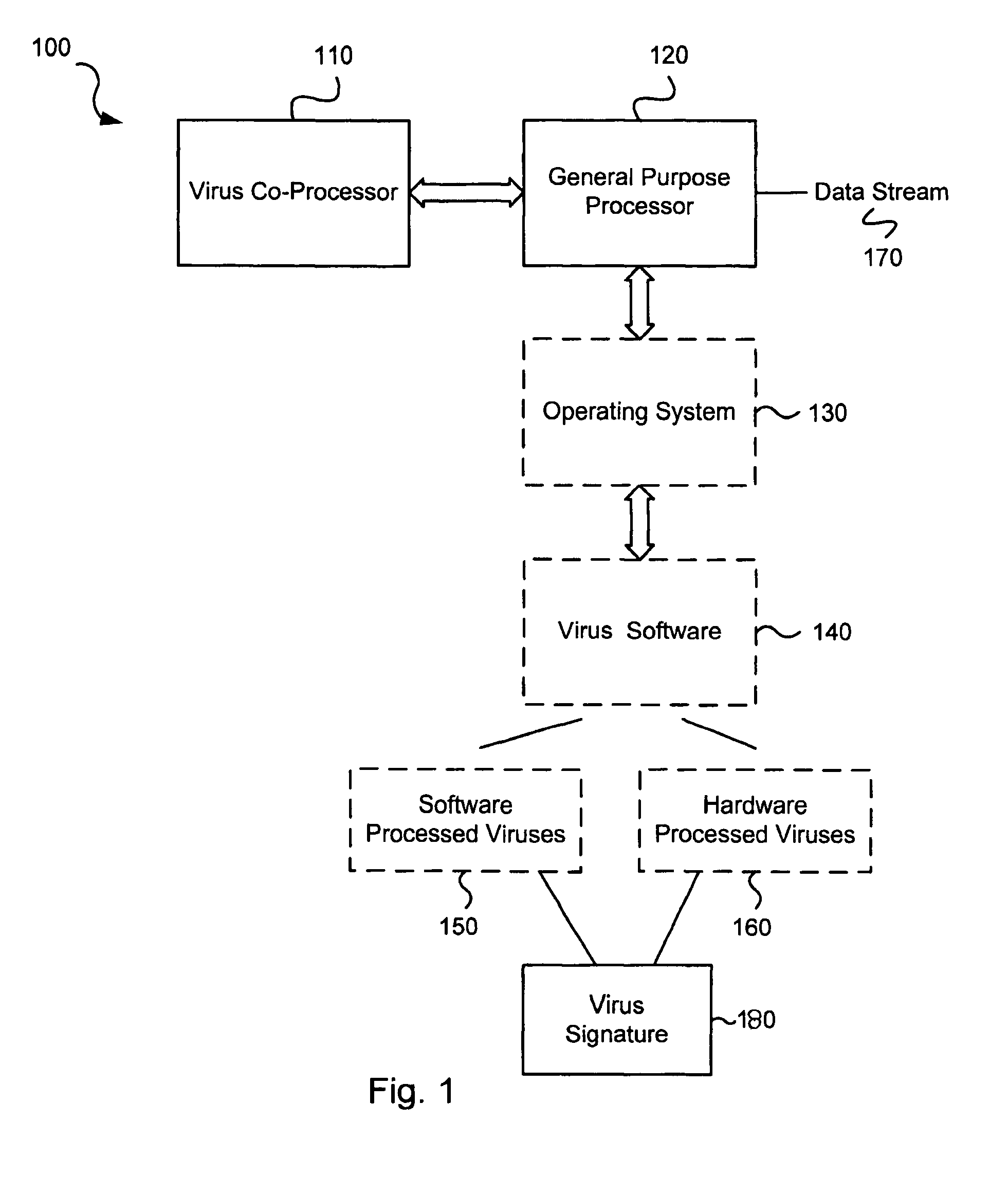
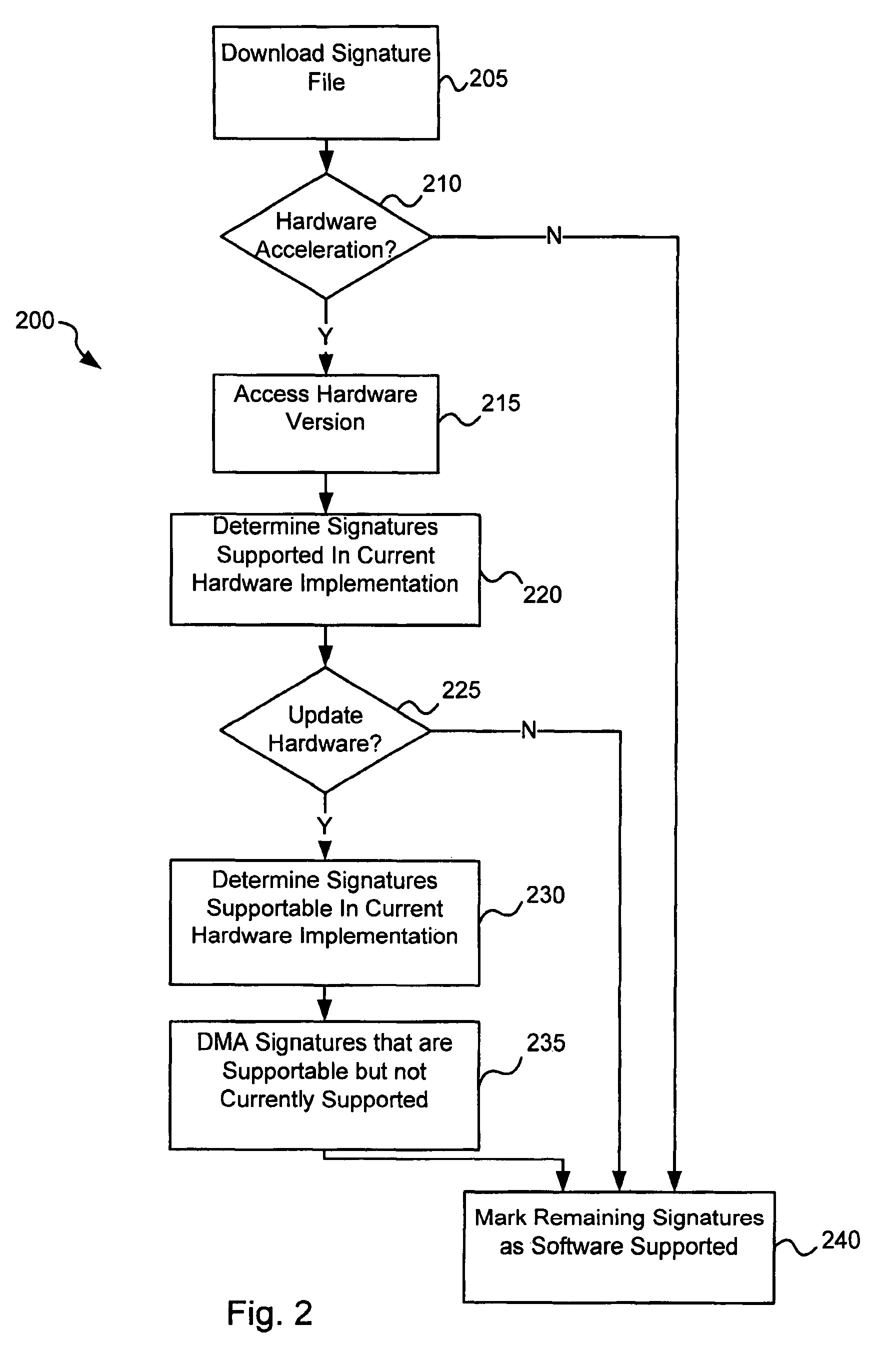
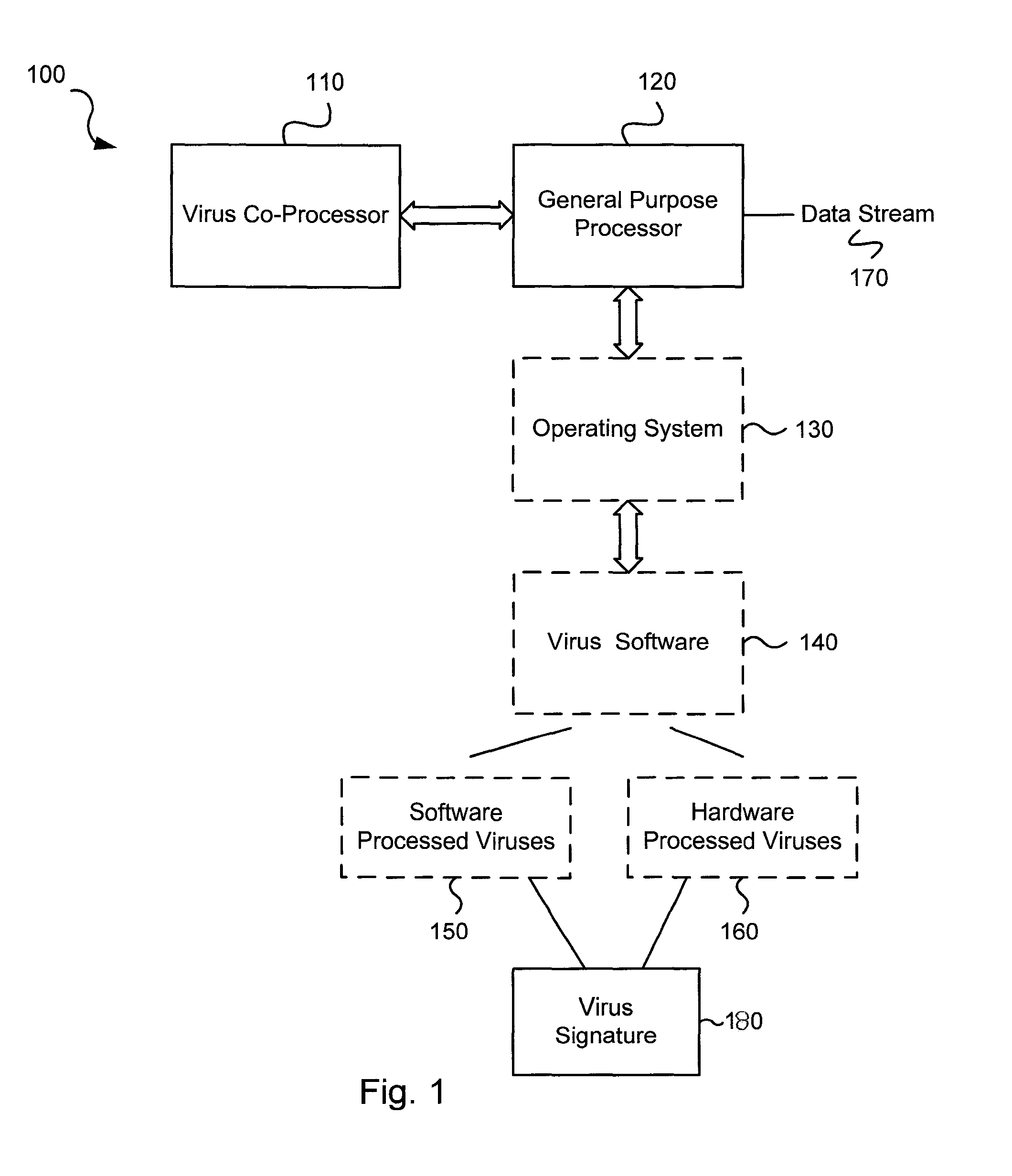
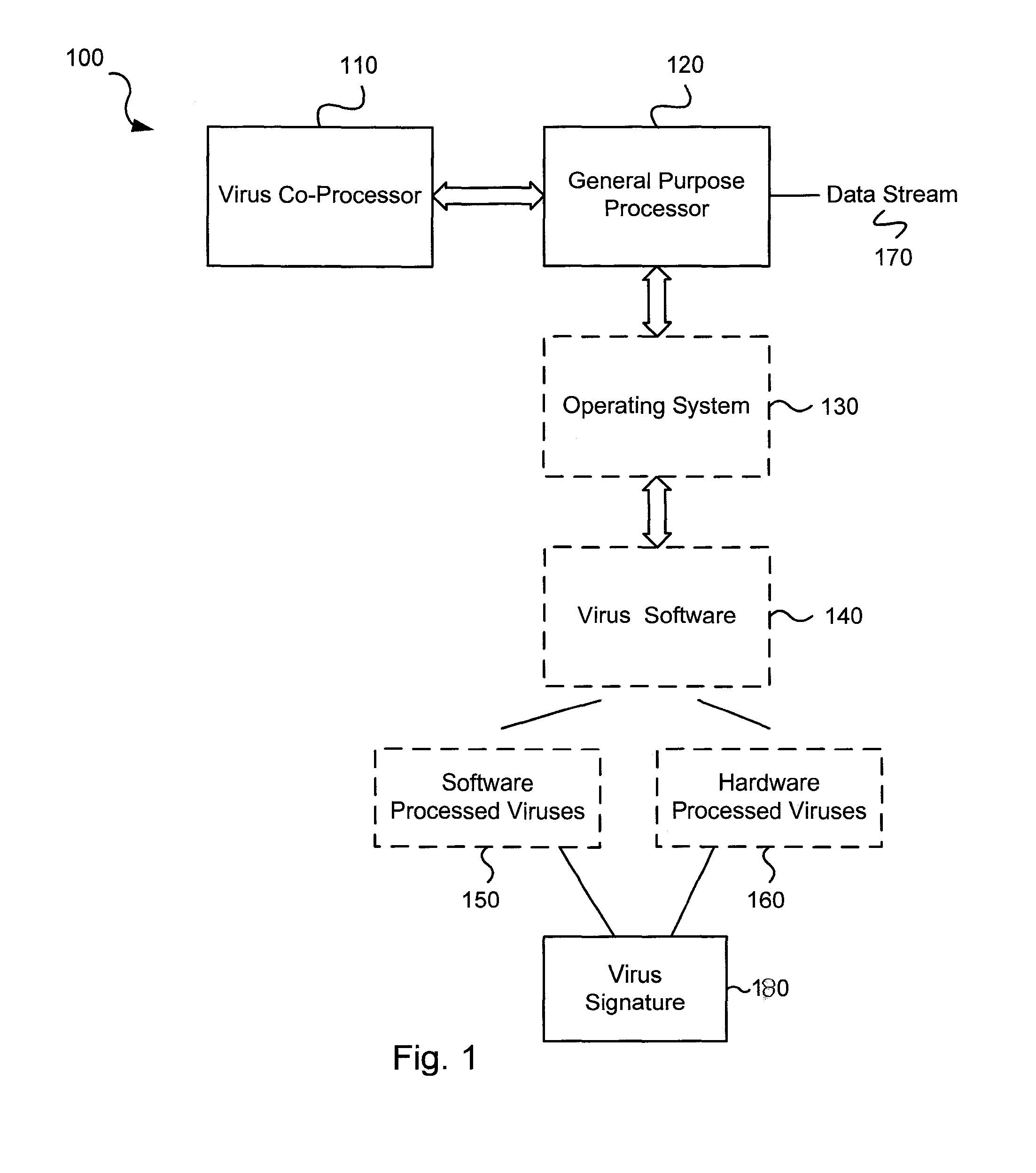
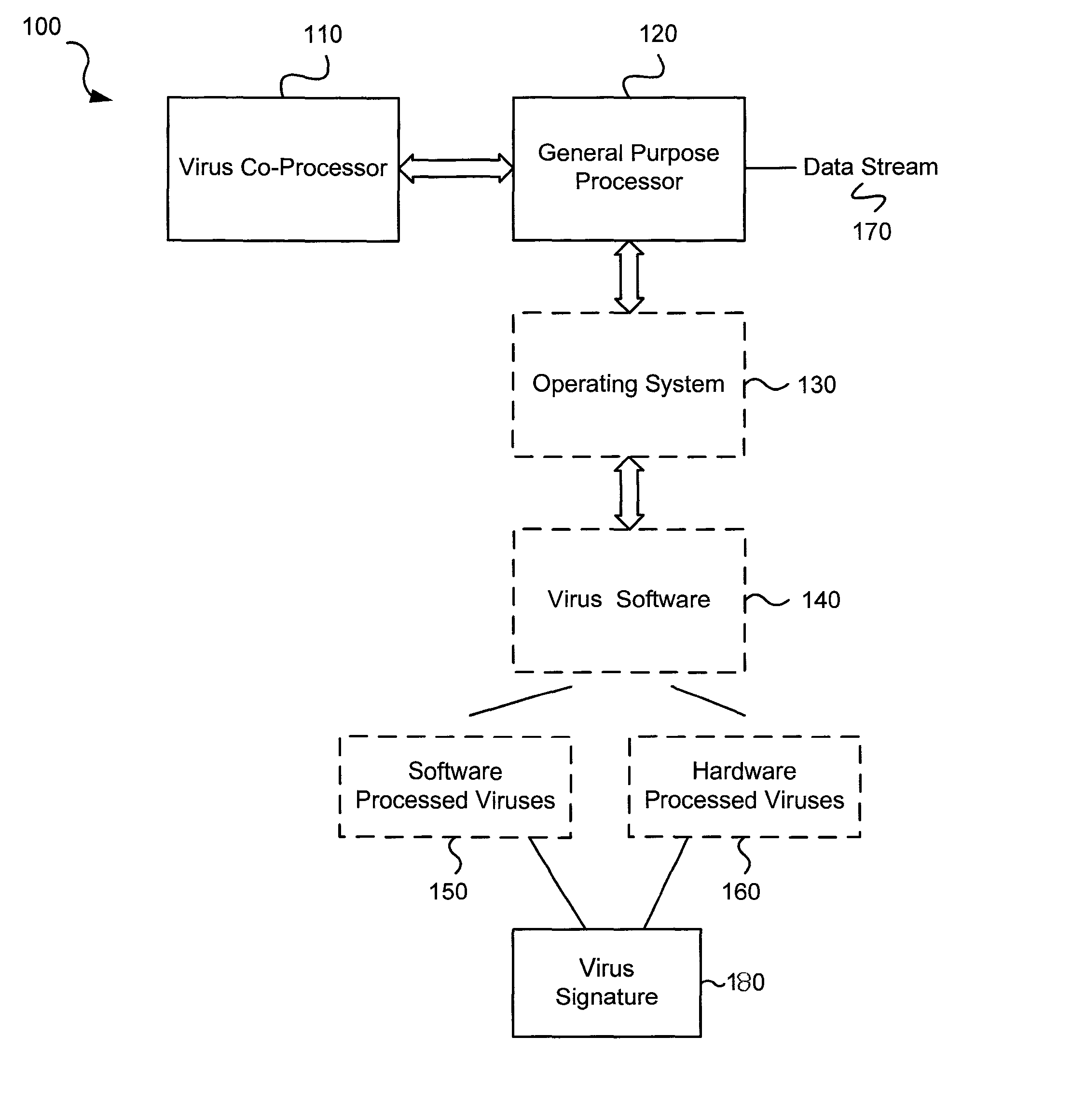
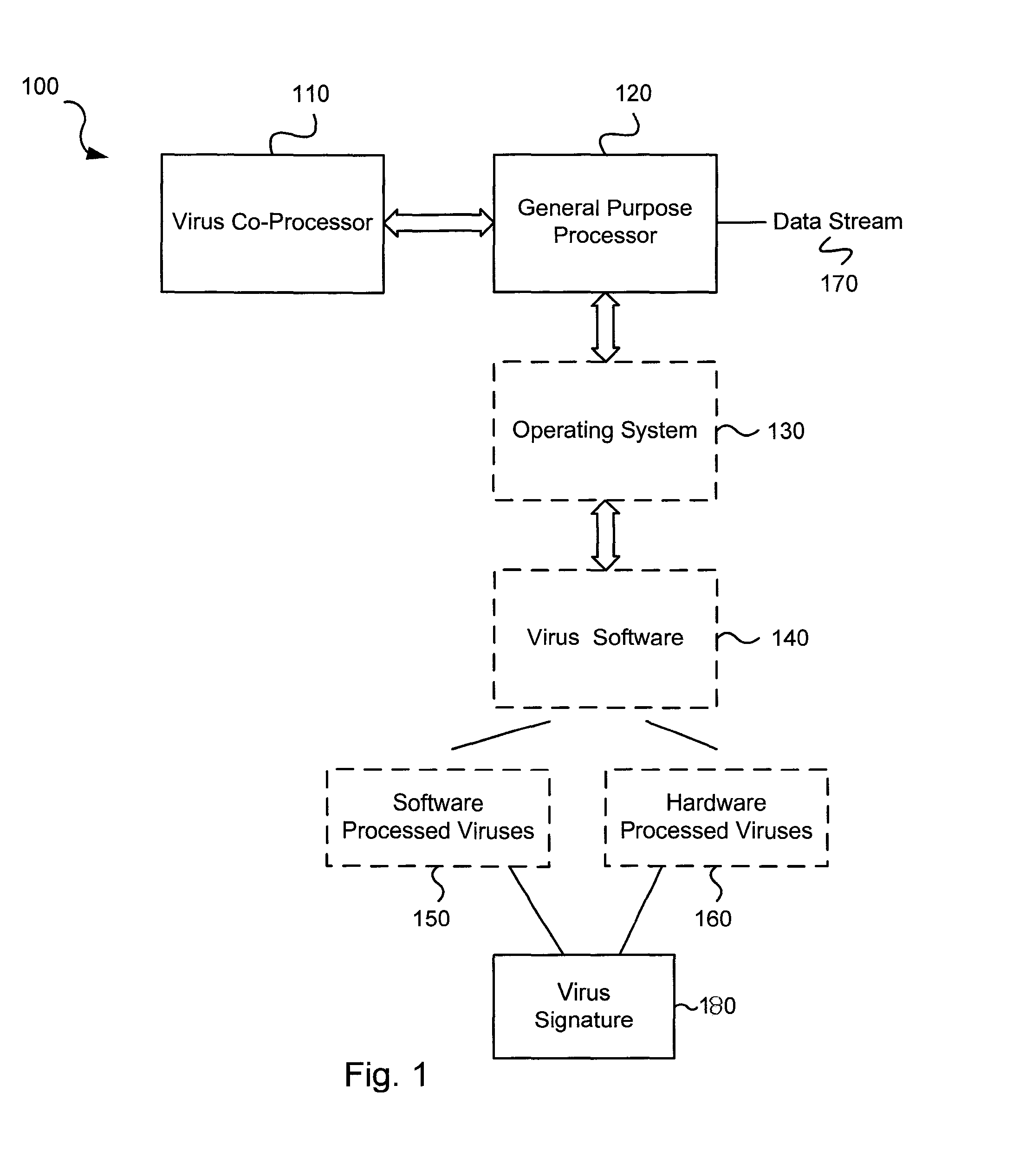
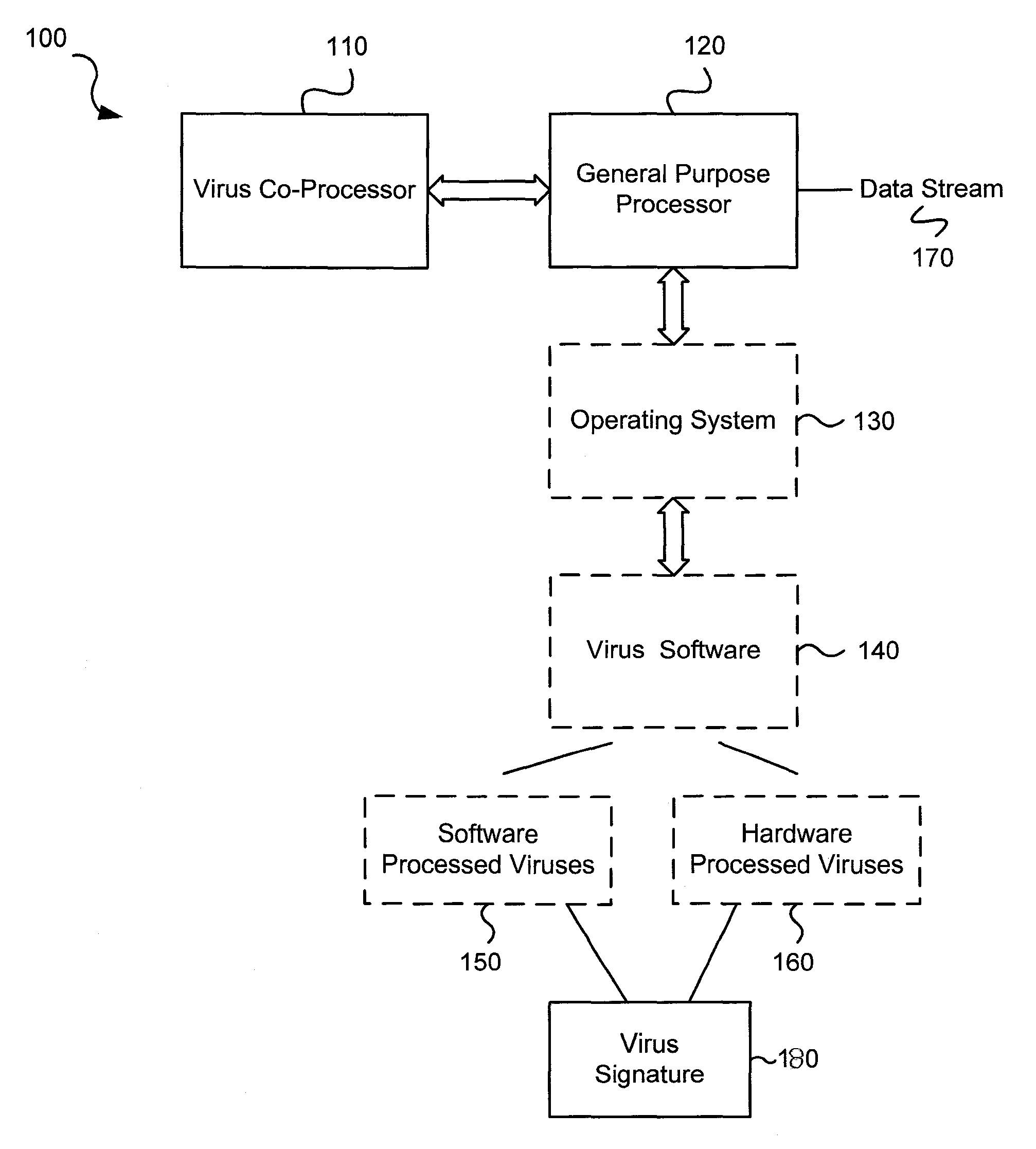
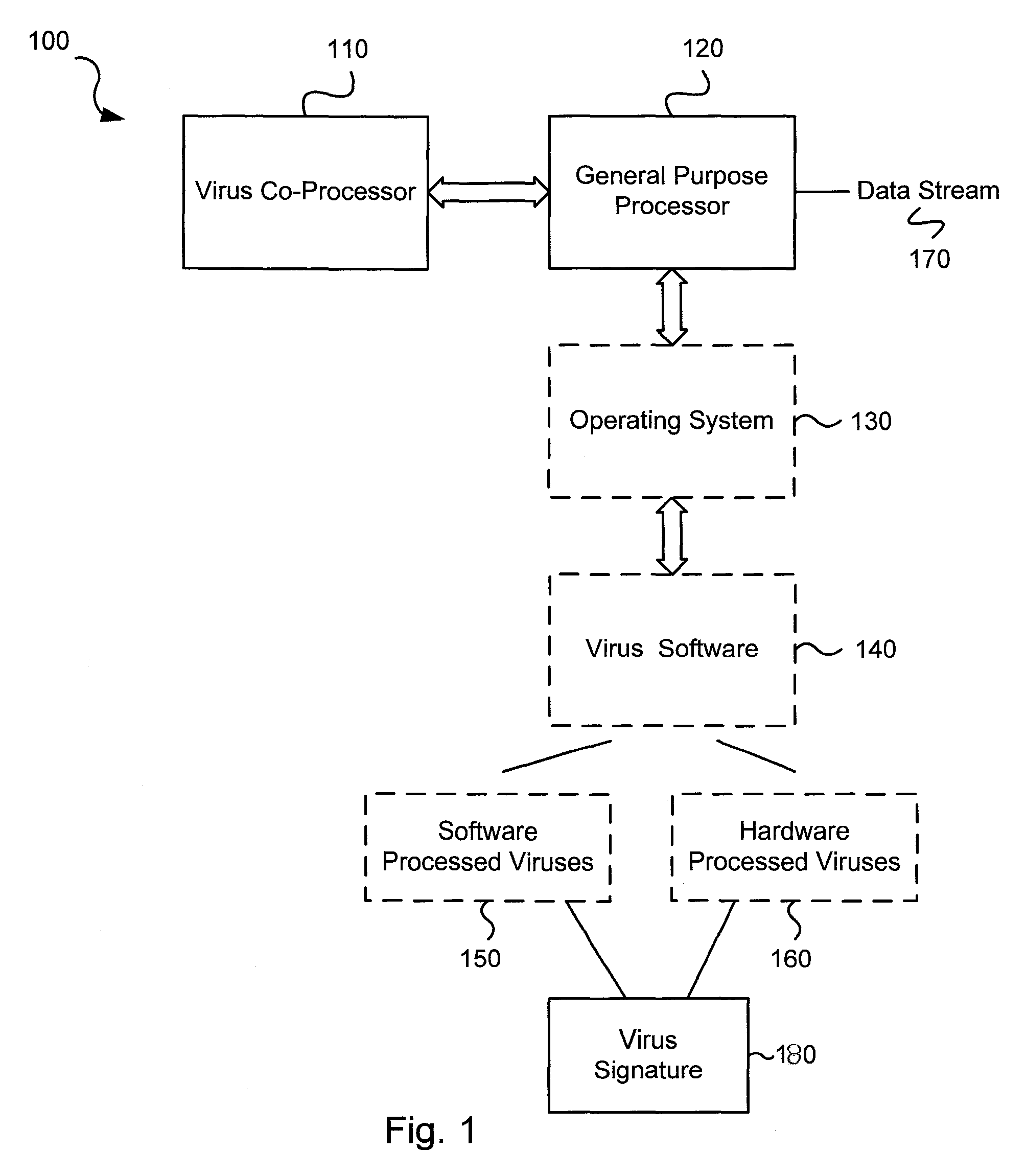
Circuits and methods for efficient data transfer in a virus co-processing system
ActiveUS20090044273A1Memory architecture accessing/allocationMemory loss protectionGeneral purposeData segment
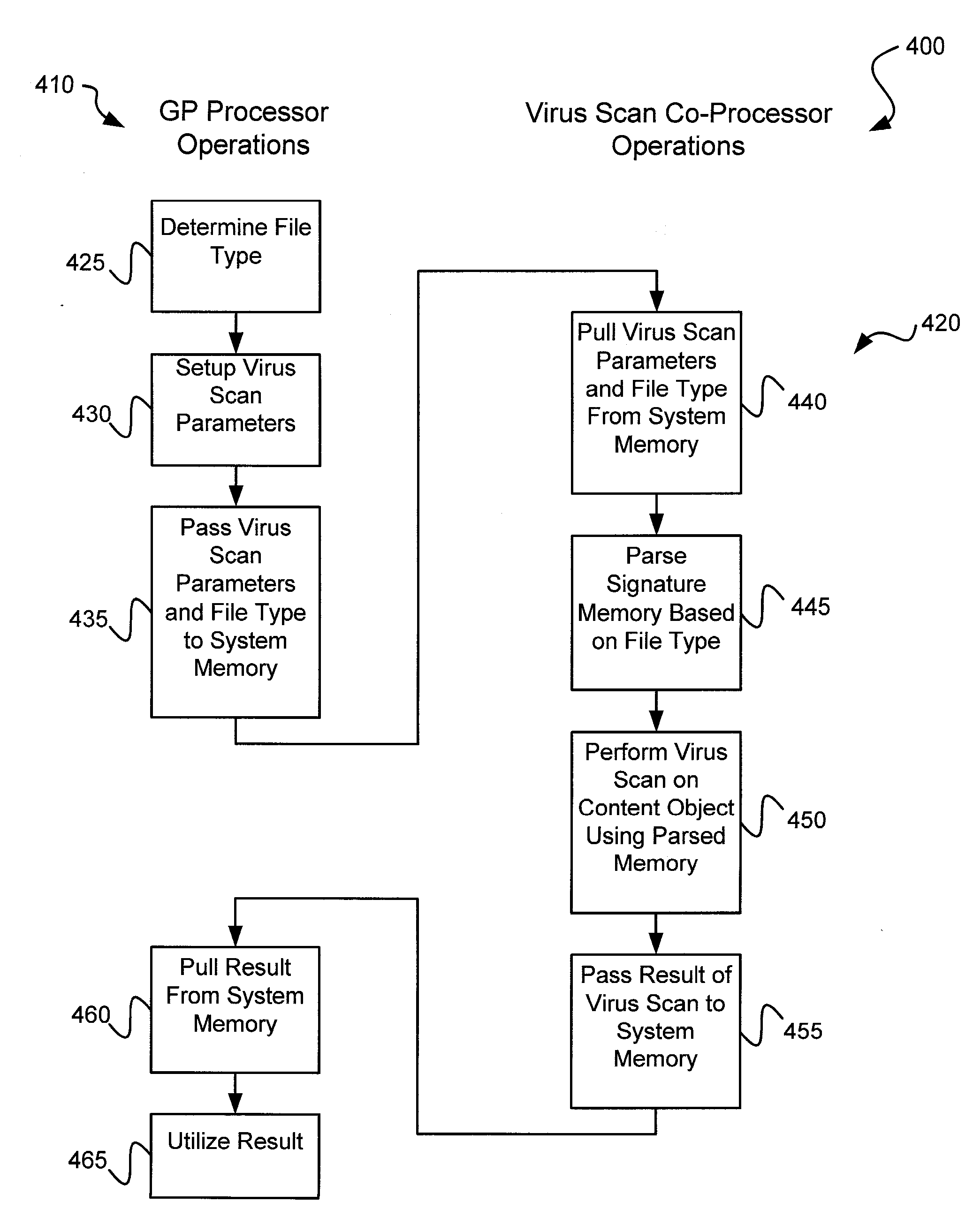
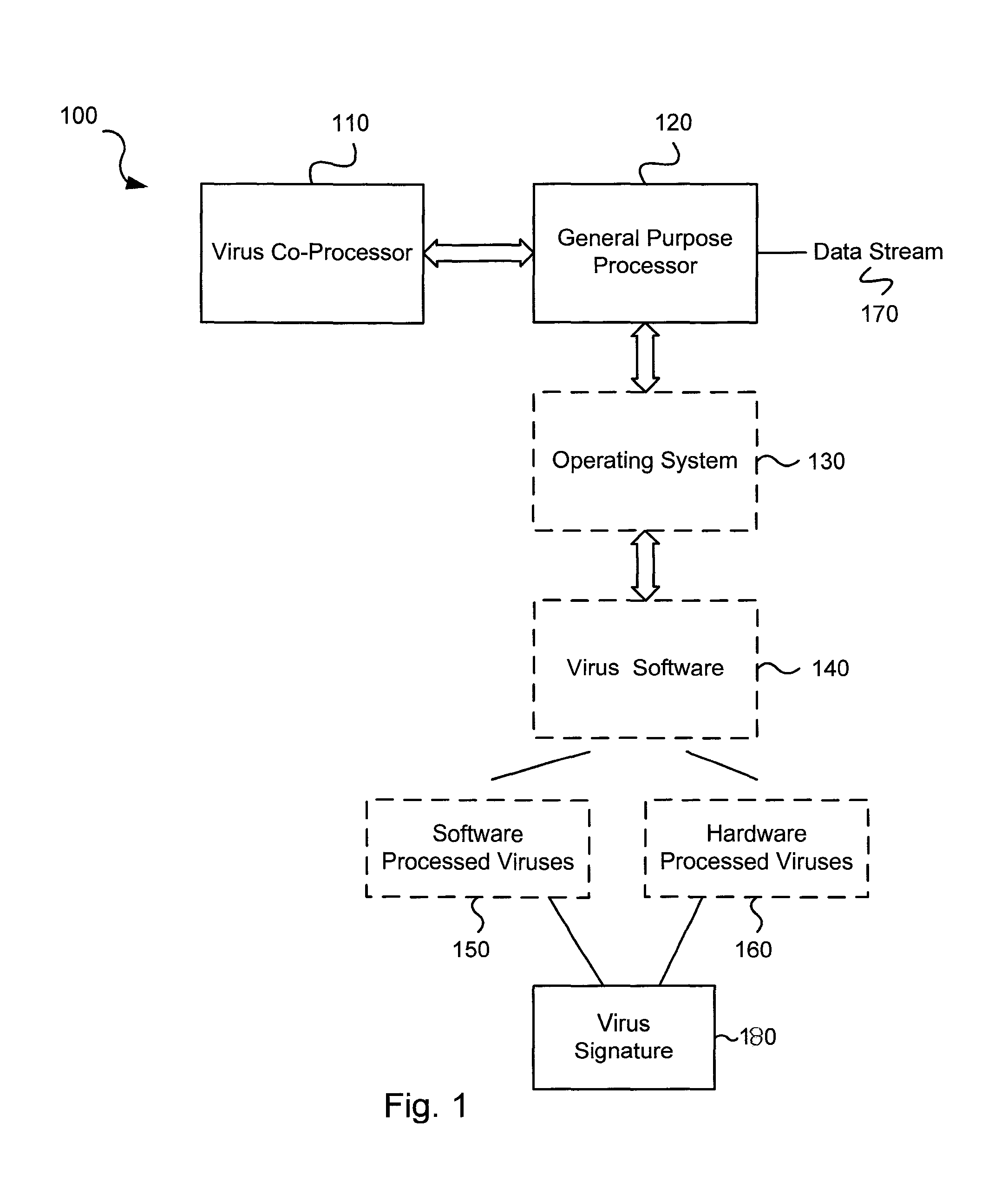
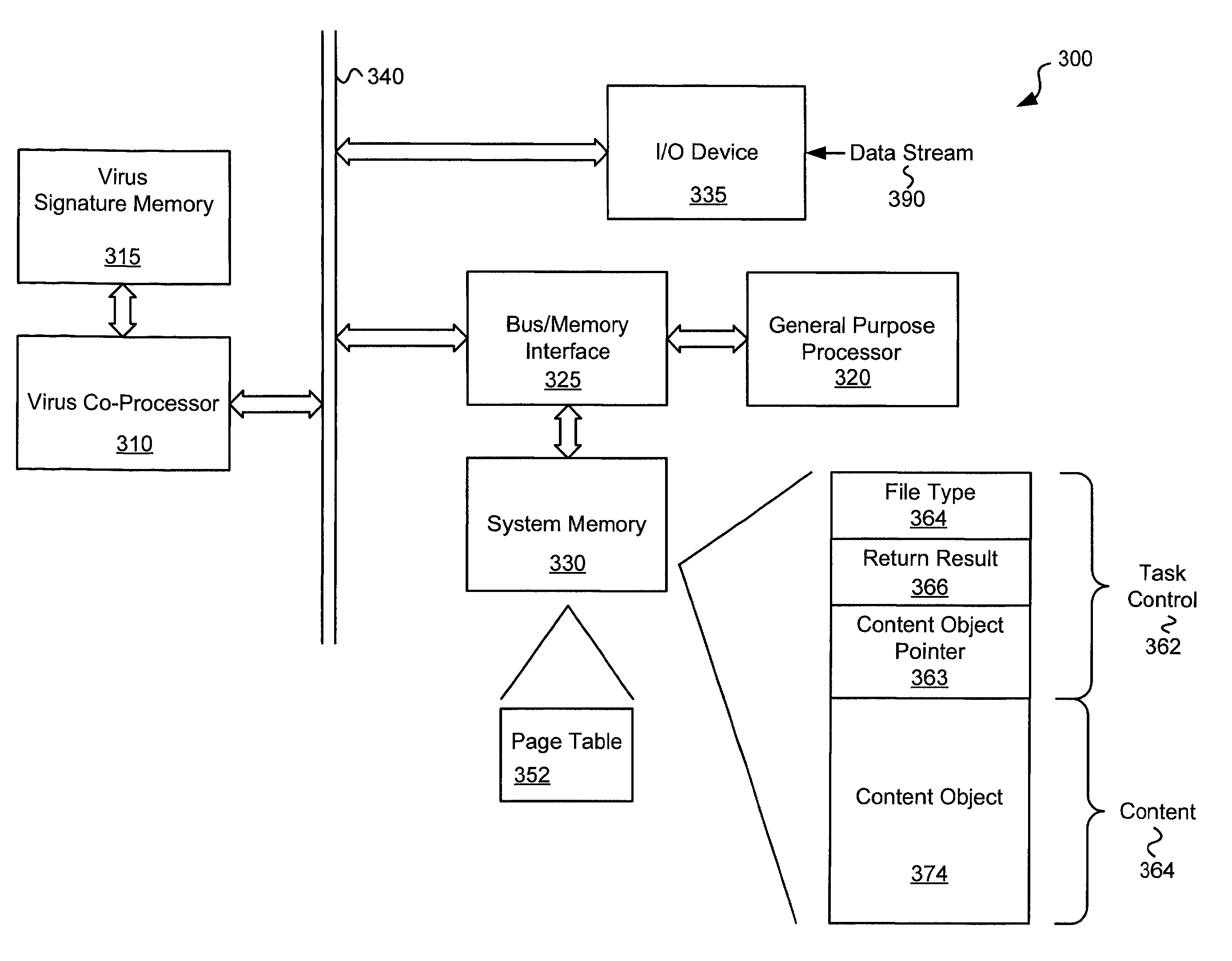
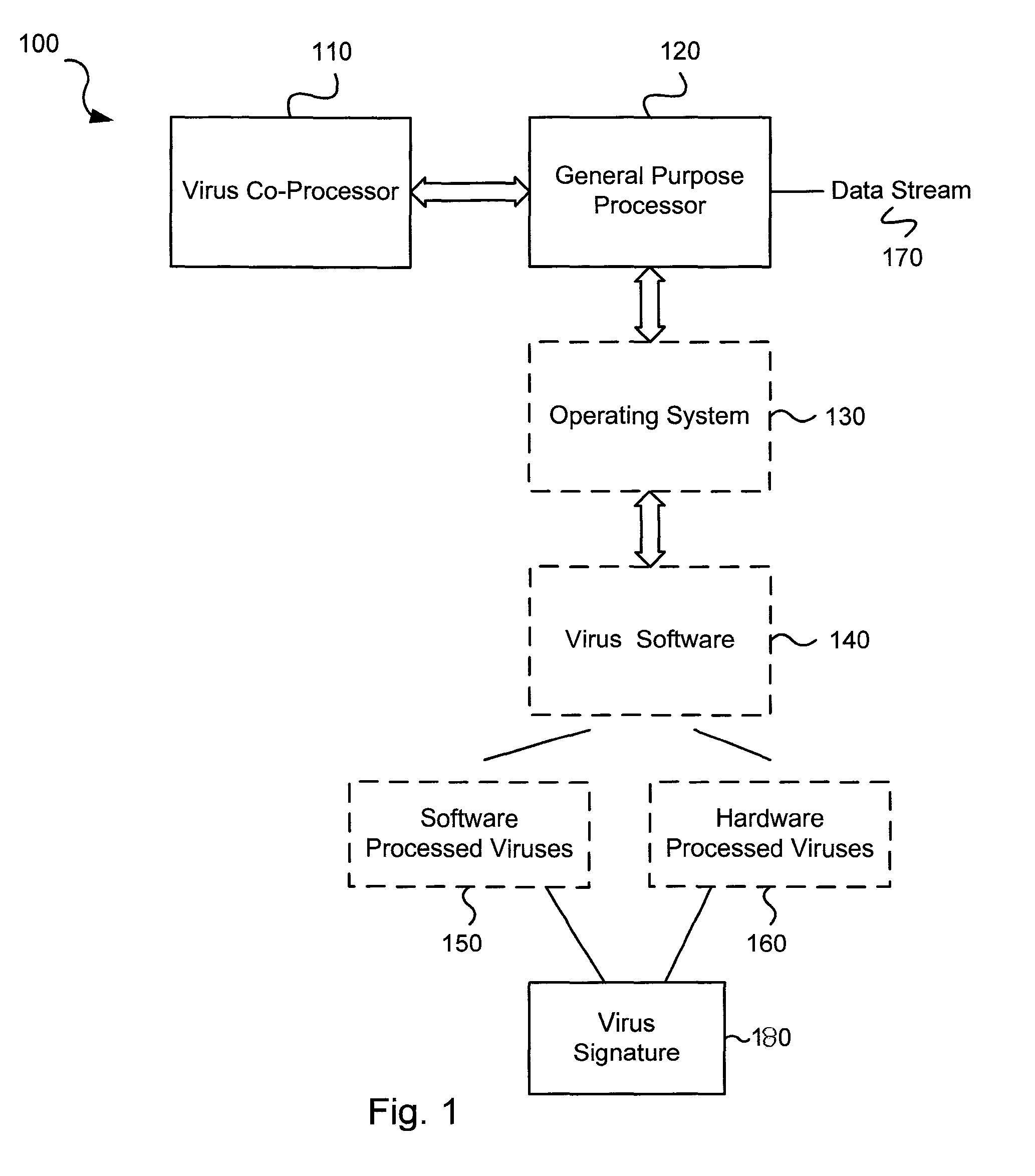
Various embodiments of the present invention circuits and methods for improved virus processing. As one example, such methods may include providing a system memory, a general purpose processor and a virus co processor. The methods further include receiving a data segment at the general purpose processor, and storing the data segment to the system memory using virtual addresses. The date segment is accessed from the system memory by the virus co processor using the virtual addresses. The virus co processor then scans the date segment for viruses and returns results.
Owner:FORTINET
Circuits and methods for operating a virus co-processor
ActiveUS8375449B1Memory loss protectionError detection/correctionInstruction memoryParallel computing
Various embodiments of the present invention provide circuits and methods for improved virus processing. As one example, a virus processing system is disclosed that includes an instruction memory and a virus co-processor. The instruction memory includes a first instruction type and a second instruction type intermixed. The virus co-processor is communicably coupled to the instruction memory, and includes at least a first instruction pipe and a second instruction pipe. The first instruction pipe is operable to execute the first instruction type, and the second instruction pipe is operable to execute the second instruction type.
Owner:FORTINET
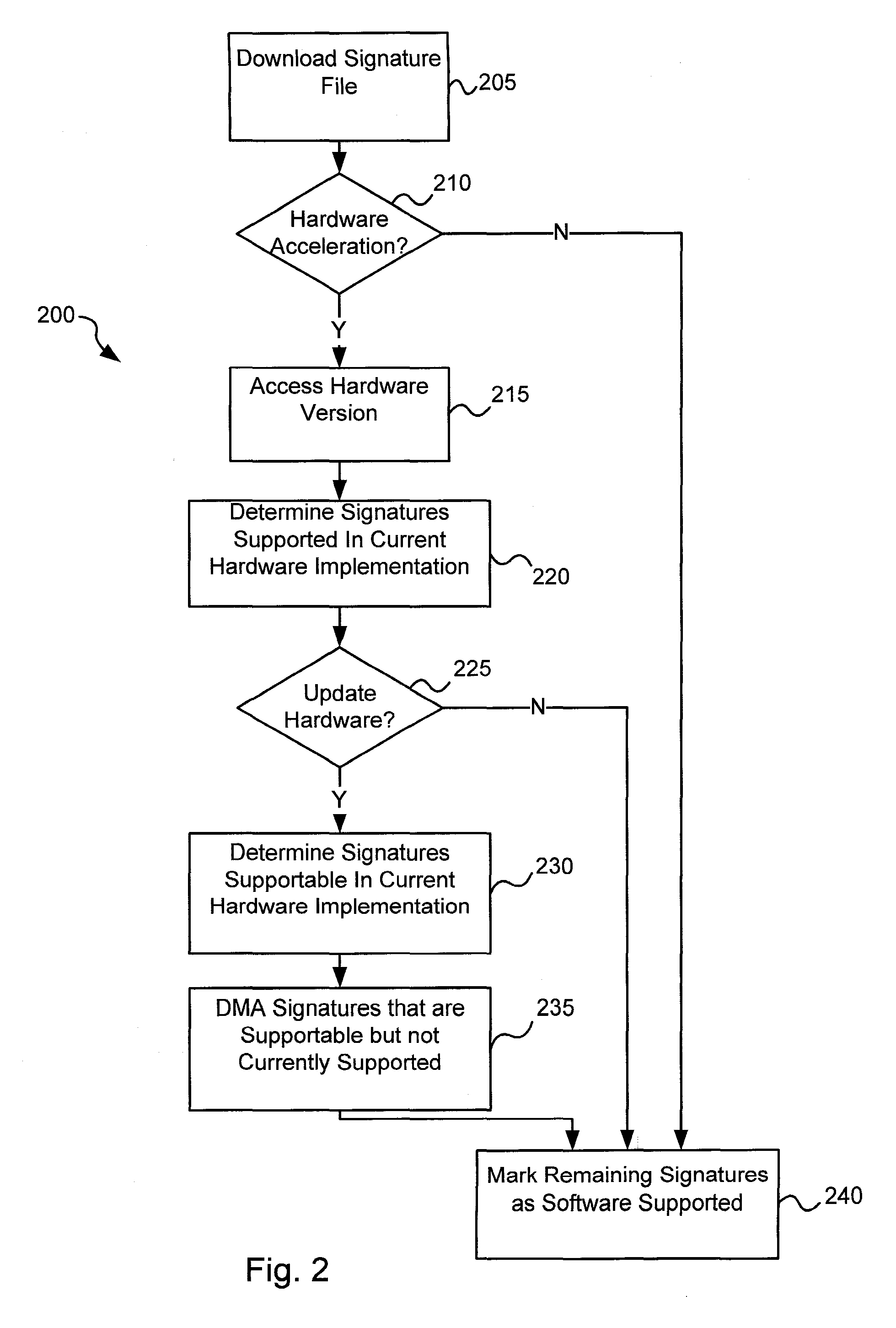
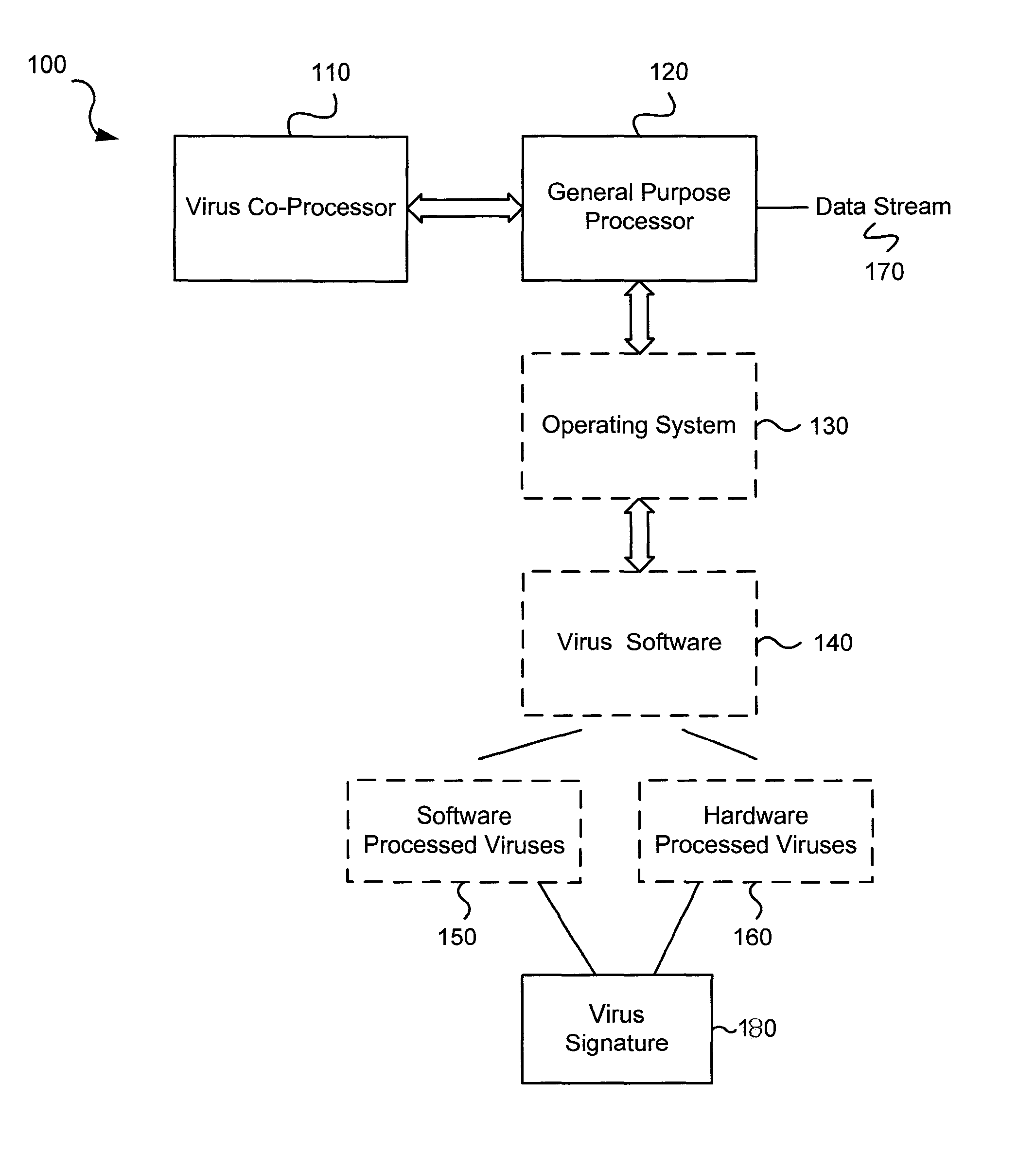
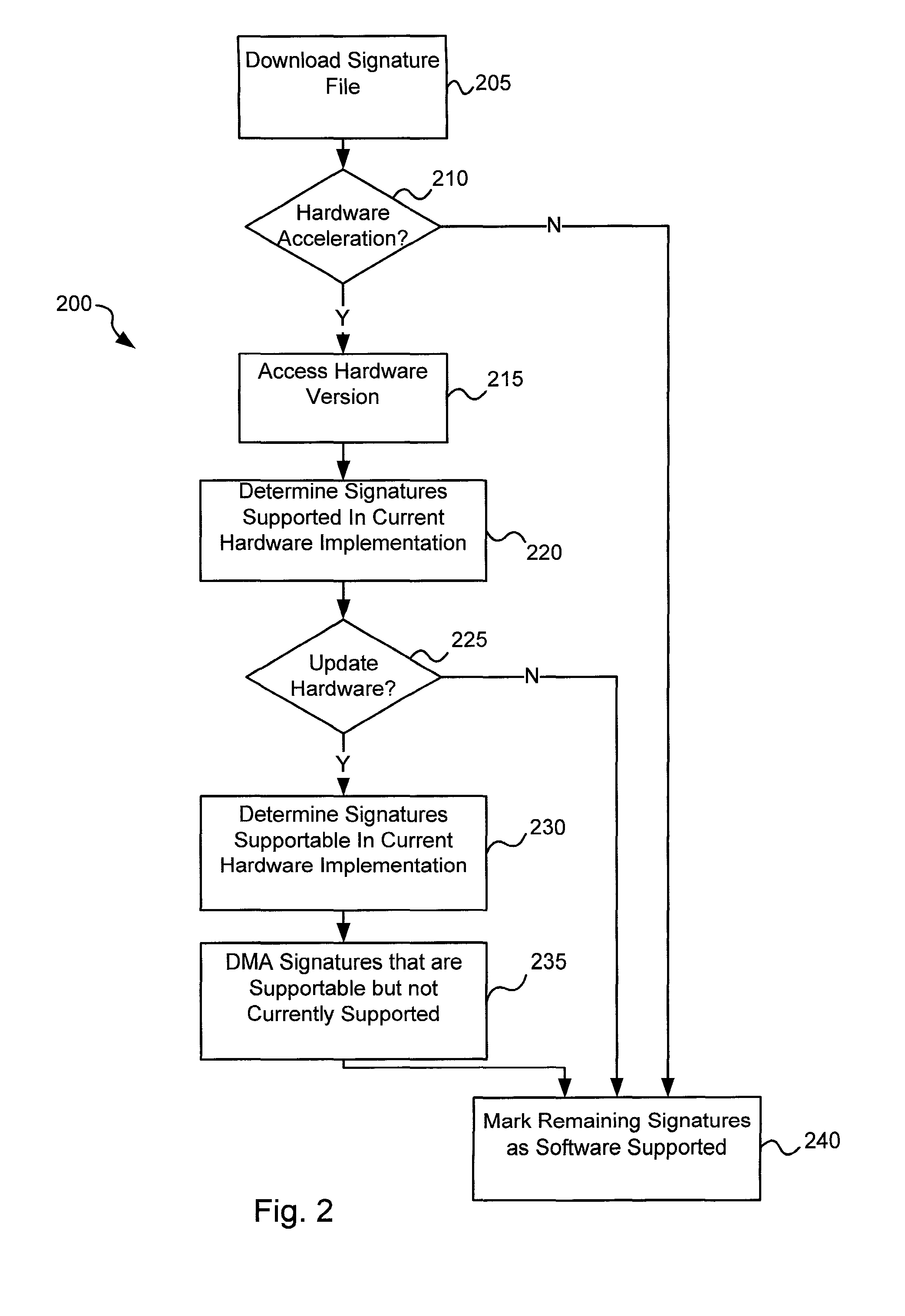
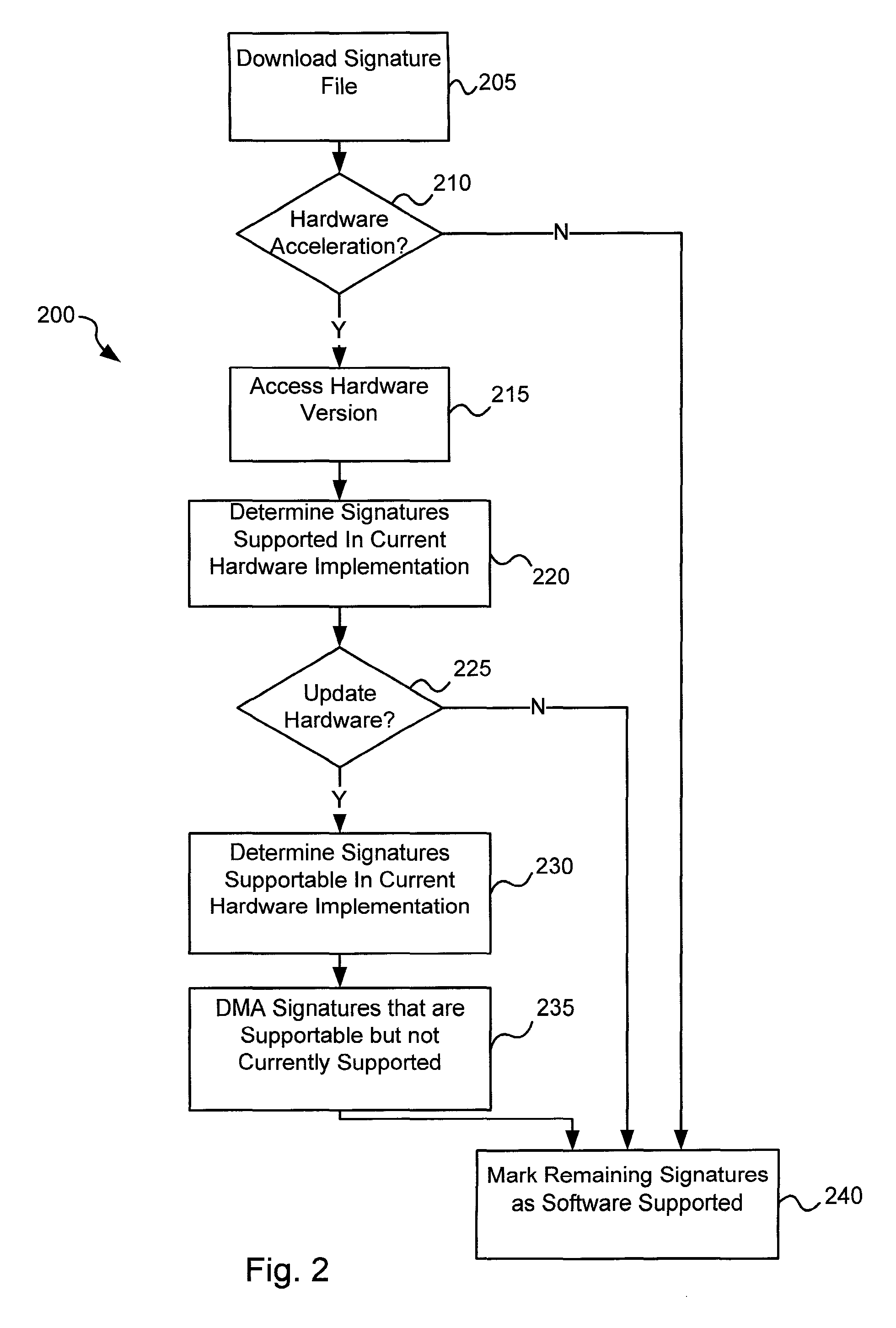
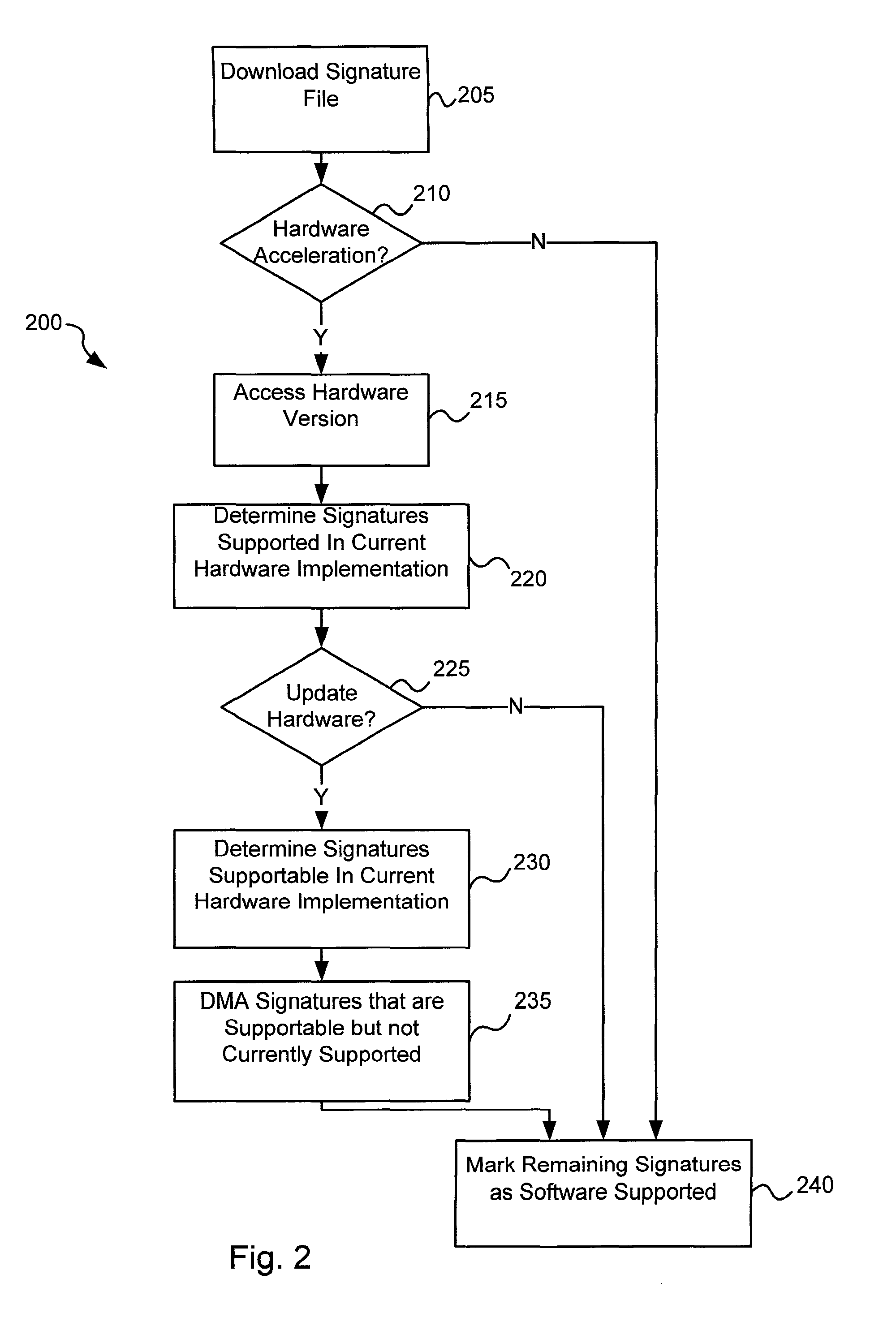
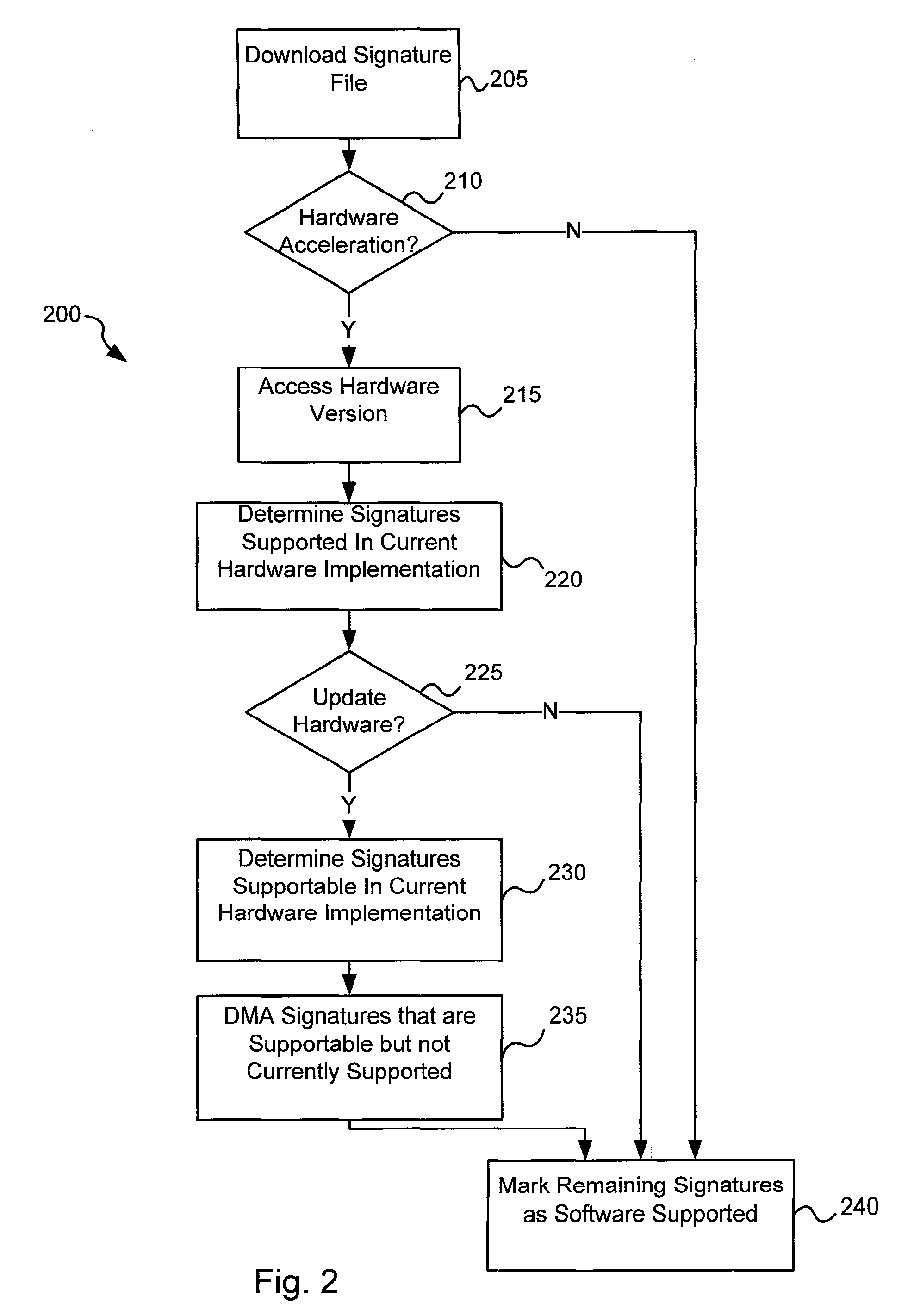
Software-hardware partitioning in a virus processing system
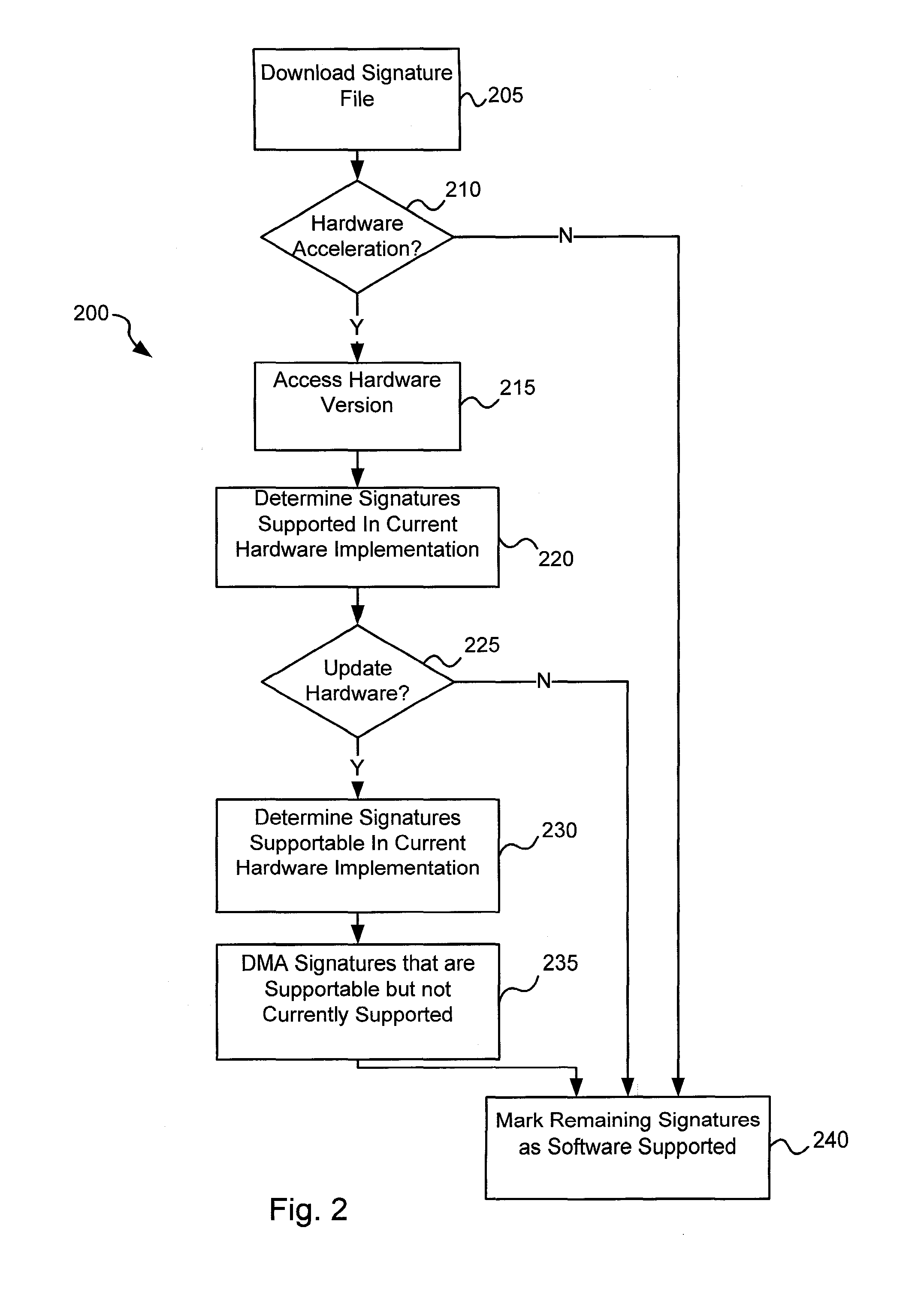
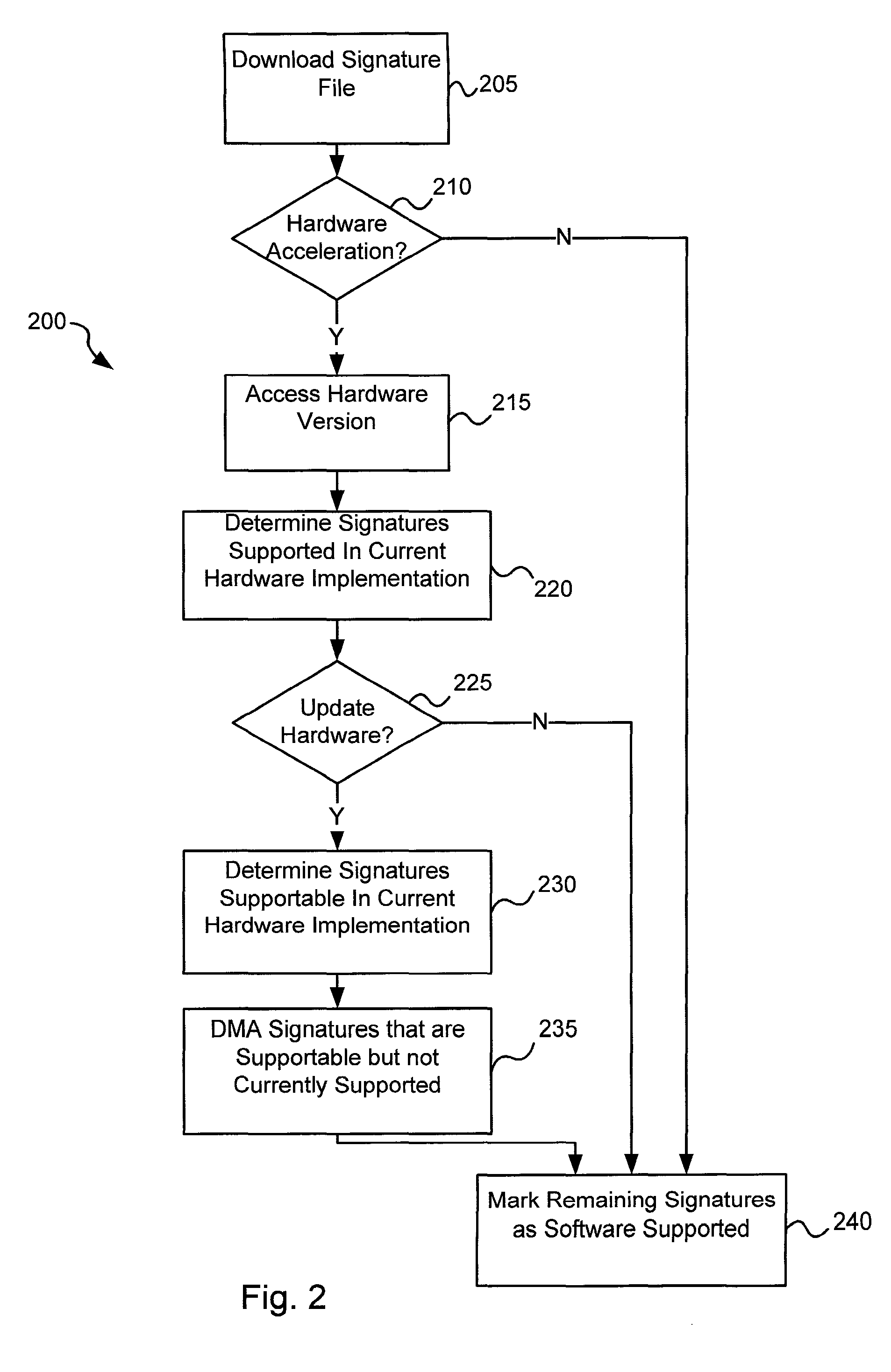
Various embodiments of the present invention provide circuits and methods for improved virus processing. As one example, a method for virus processing is disclosed that includes providing a first memory that includes a first set of virus signatures, and a second memory that includes a second set of virus signatures. In addition, a virus co-processor and a general purpose processor are provided. The virus co-processor is communicably coupled to the first memory, and the general purpose processor is communicably coupled to the virus co-processor and to the second memory. A subset of the second set of virus signatures that is not included in the first set of virus signatures is determined. The subset of the second set of virus signatures is processed on the general processor, and the first set of the virus signatures is processed on the virus co-processor.
Owner:FORTINET
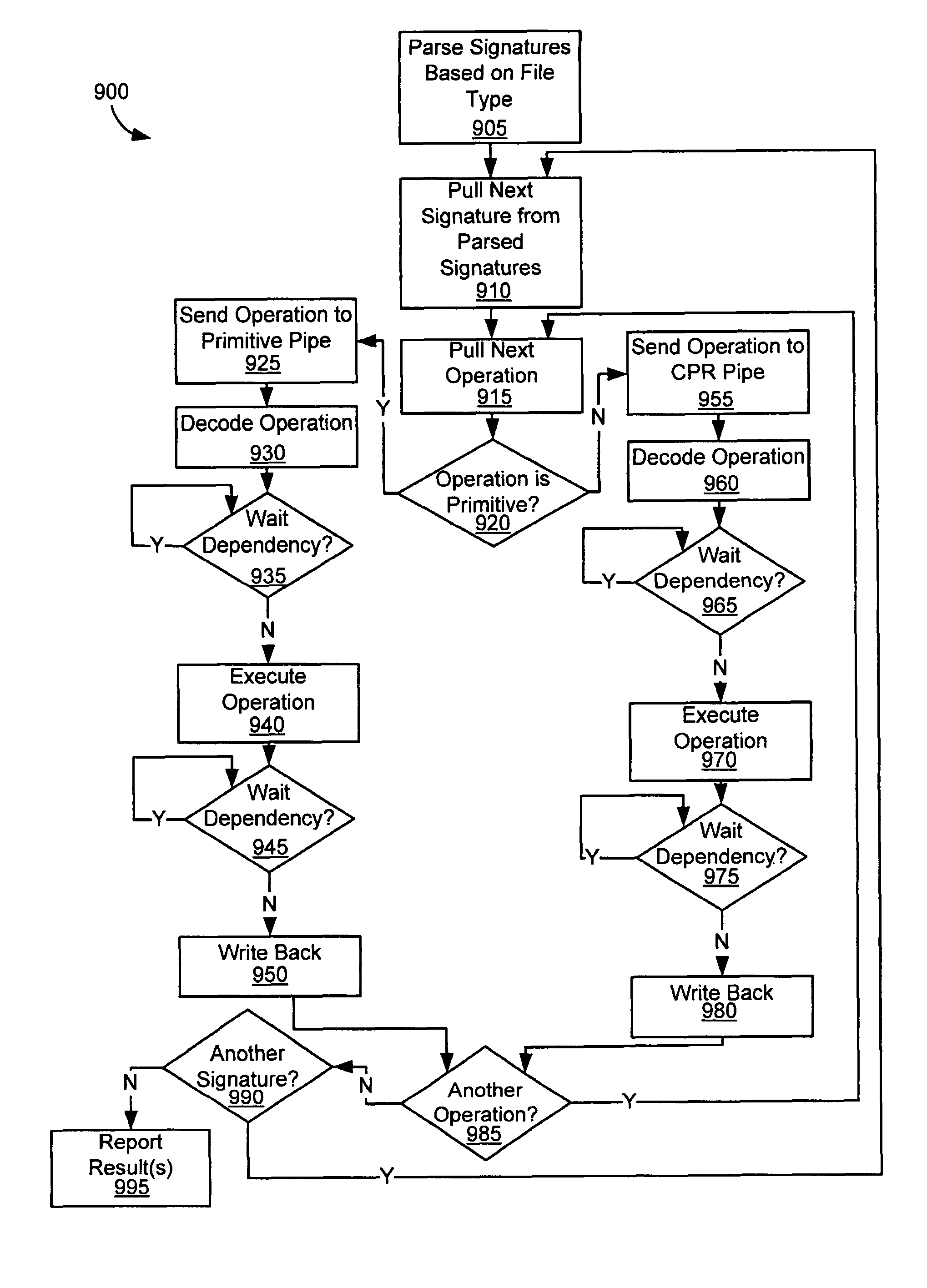
Operation of a dual instruction pipe virus co-processor
Owner:FORTINET
Circuits and methods for efficient data transfer in a virus co-processing system
ActiveUS8286246B2Memory architecture accessing/allocationMemory loss protectionGeneral purposeData segment
Various embodiments of the present invention provide circuits and methods for improved virus processing. As one example, such methods may include providing a system memory, a general purpose processor and a virus co-processor. The methods further include receiving a data segment at the general purpose processor, and storing the data segment to the system memory using virtual addresses. The data segment is accessed from the system memory by the virus co-processor using the virtual addresses. The virus co-processor then scans the data segment for viruses and returns a result.
Owner:FORTINET
Virus co-processor instructions and methods for using such
ActiveUS8079084B1Memory loss protectionUnauthorized memory use protectionLoad instructionInstruction unit
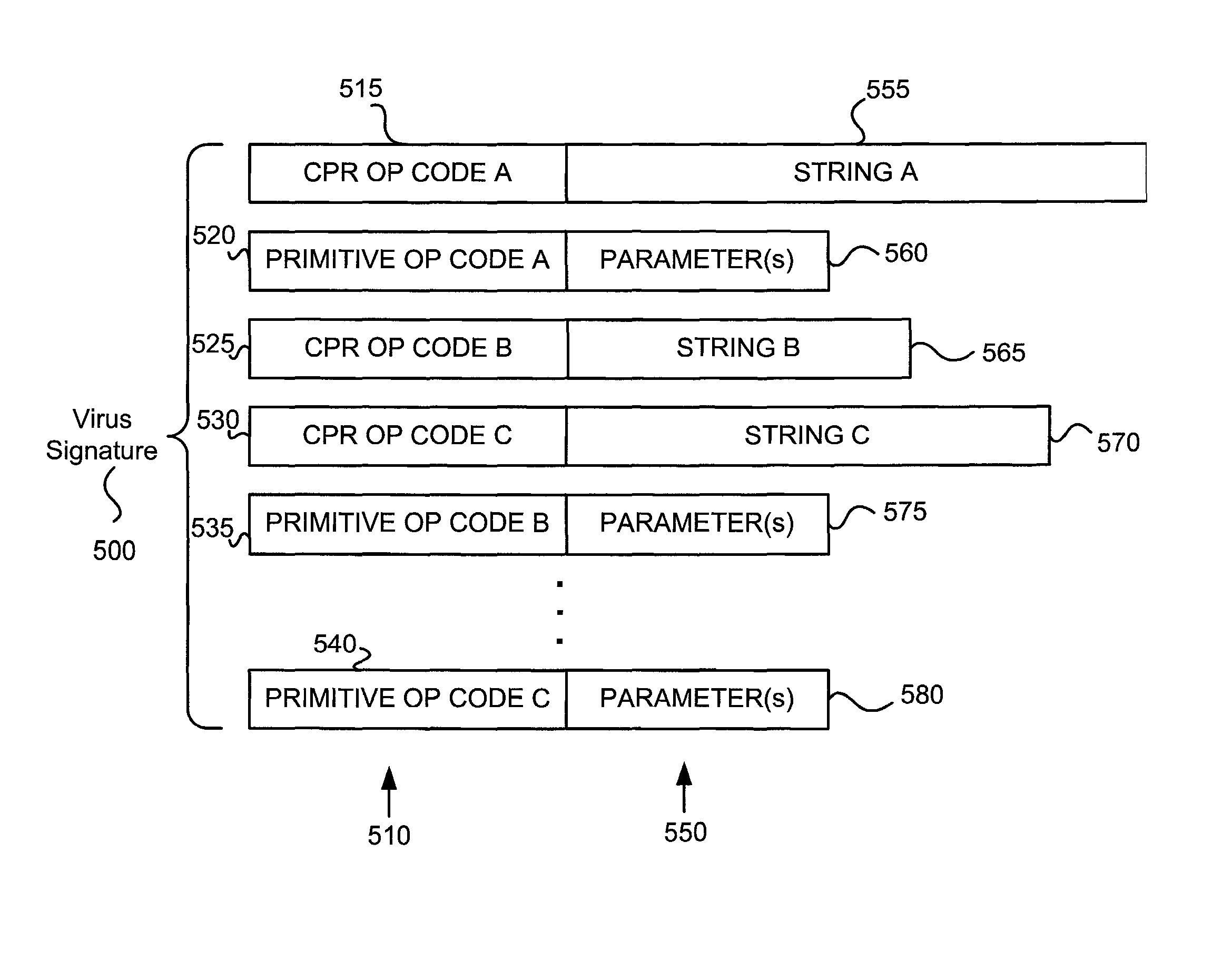
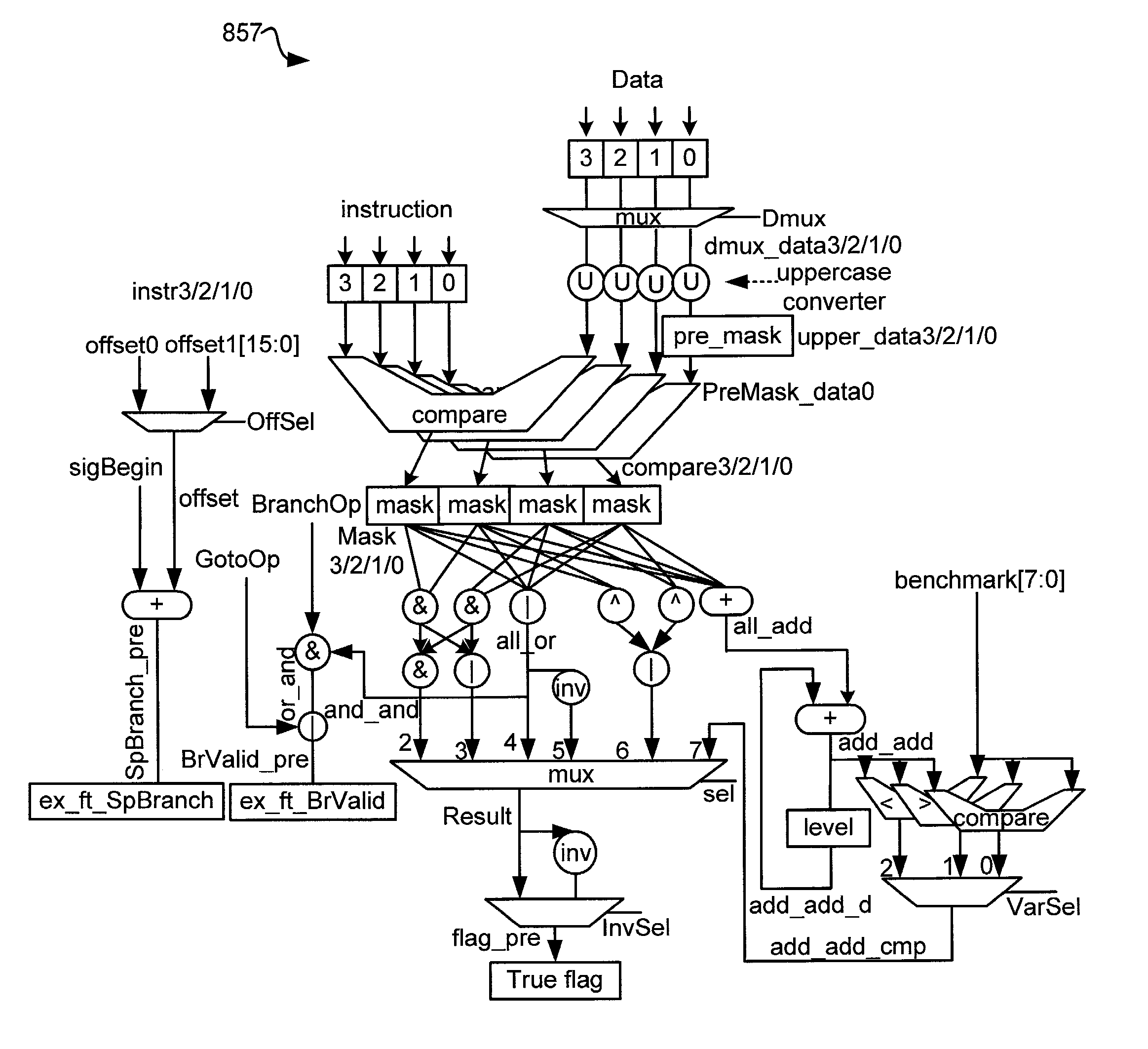
Various embodiments of the present invention provide elements that may be utilized for improved virus processing. As one example, a computer readable medium is disclosed that includes a virus signature compiled for execution on a virus co-processor. The virus signature includes at least one primitive instruction and at least one CPR instruction stored at contiguous locations in the computer readable medium. The CPR instruction is one of an instruction set that includes, but is not limited to: a compare string instruction, compare buffer instruction; perform checksum instruction; a seek instruction; and a test instruction. The primitive instruction may be, but is not limited to, an add instruction, a branch instruction, a jump instruction, a load instruction, a move instruction, a logic AND instruction, a logic OR instruction, and / or a logic XOR instruction.
Owner:FORTINET
Virus co-processor instructions and methods for using such
ActiveUS8239950B1Memory loss protectionUnauthorized memory use protectionGeneral purposeComputer hardware
Circuits and methods are provided for detecting, identifying and / or removing undesired content. According to one embodiment, a method for virus processing is provided. A general purpose processor, communicably coupled to a virus processing hardware accelerator, receives a data segment. The general purpose processor causes the data segment to be stored to a first memory. The general purpose processor directs the virus processing hardware accelerator, communicably coupled to the first memory and to the second memory, to perform a virus scan of the data segment based on one or more virus signatures stored in a second memory. The first memory includes a first virus signature compiled for execution on the general purpose processor. The second memory includes virus signatures, each of which include at least one primitive instruction and at least one Content Pattern Recognition (CPR) instruction stored at contiguous locations, compiled for execution by the virus processing hardware accelerator.
Owner:FORTINET
Air decontamination equipment
InactiveUS20110223057A1Most efficientReduce volatilityGas treatmentDispersed particle separationAir decontaminationVirus processing
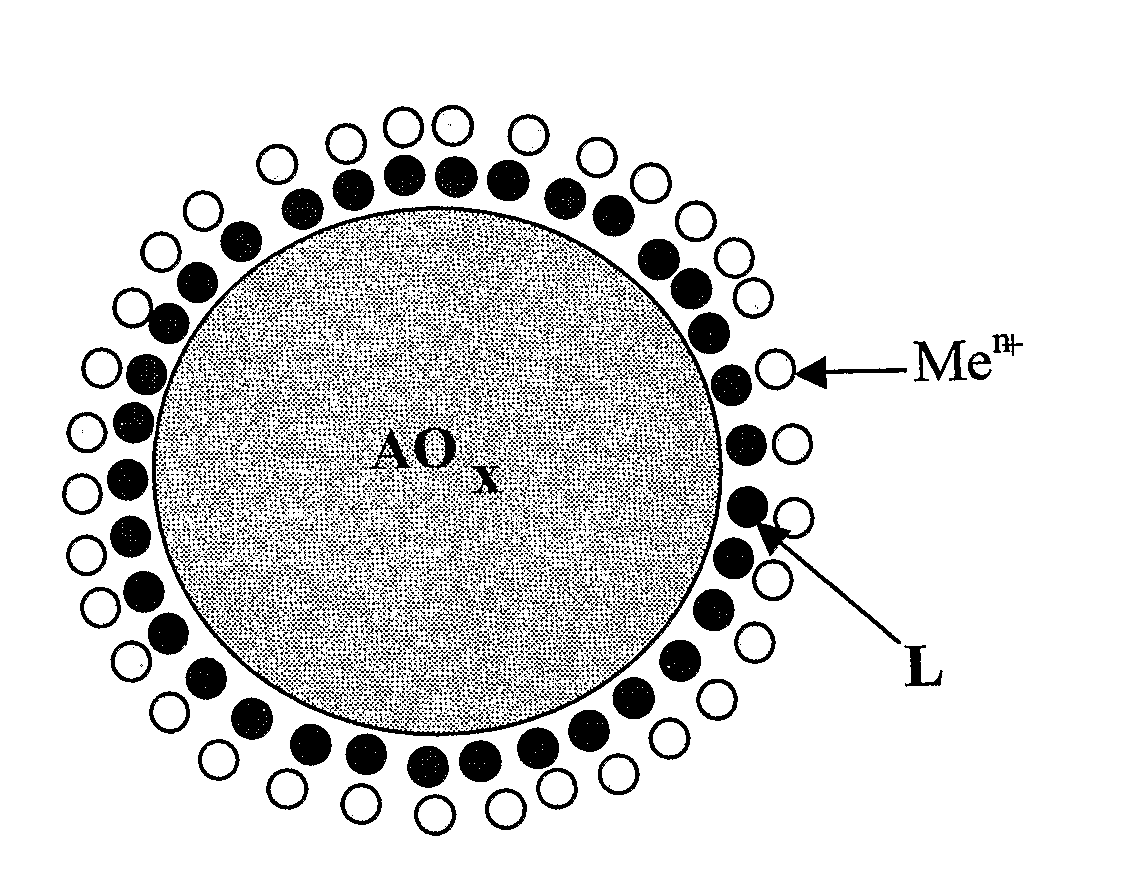
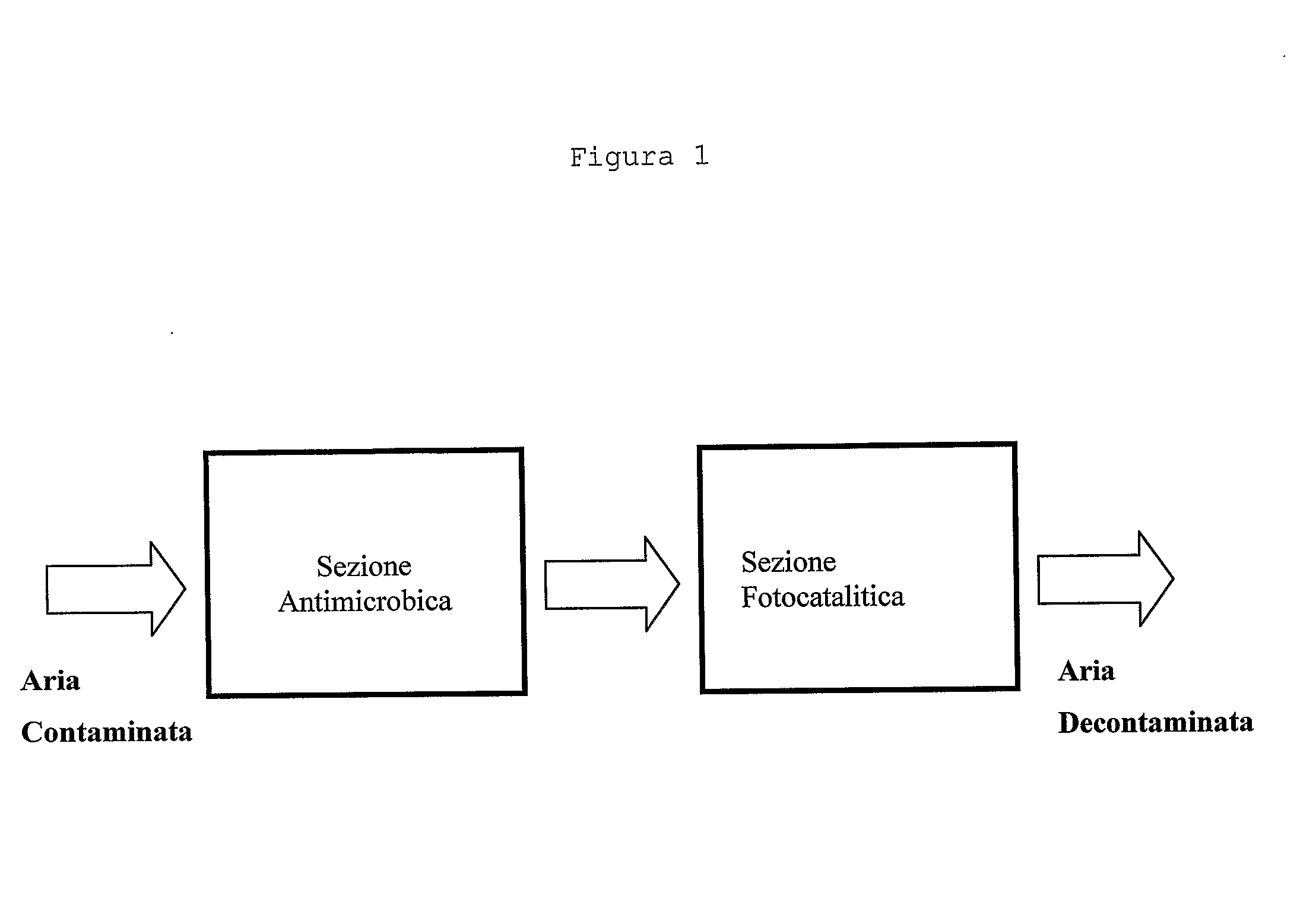
The present invention relates to an air decontamination equipment, from both odours or pollutants, and bacterial or viral loads. More particularly, the present invention relates to a decontaminating equipment (1) for the treatment of air, comprising a shell (2) which is divided in a first and a second compartments (3, 4), which are arranged in a contiguous position in any sequence order, in the second of said compartments (3, 4) suction means (6) being arranged, in which one of said first and second compartments (3, 4) is for the antibacterial / antiviral treatment of air, and one of said first and second compartments (3, 4) is for the photocatalytic treatment of air, and comprises UV illumination means (9), said first and second compartments (3, 4) comprising a material with antibacterial and antiviral activity and a material with photocatalytic activity, respectively.
Owner:NM TECH NANOMATERIALS & MICRODEVICES TECH
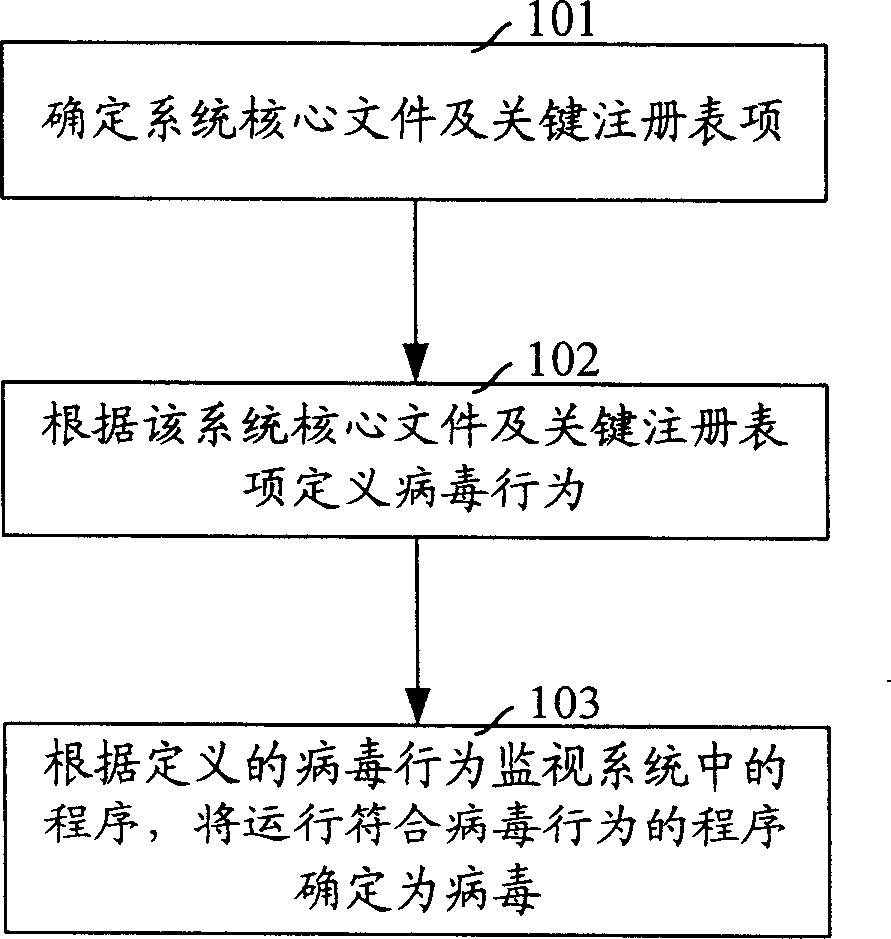
Virus processing method
InactiveCN1889004AImprove prevention capabilitiesReduce system riskDigital data processing detailsComputer scienceSystem safety
The invention discloses a virus processing method. It determines the key data relates to the system safety in the system firstly and regards the operation to the key data as the virus behavior; then to judge the working procedure. If it is the virus, then the procedure is the virus procedure. The invention can prevent the unknown virus to attack the system, so it can decrease the hazard to the system and the user data by the virus.
Owner:LENOVO (BEIJING) CO LTD
Efficient data transfer in a virus co-processing system
ActiveUS8560862B1Memory architecture accessing/allocationMemory loss protectionGeneral purposeVirtual memory
Circuits and methods are provided for detecting, identifying and / or removing undesired content. According to one embodiment, a method for virus co-processing is provided. A general purpose processor stores a data segment to a system memory of the general purpose processor using virtual addresses. A virus processing hardware accelerator coupled to the general purpose processor via an interconnect bus accesses the data segment by performing direct virtual memory addressing of the system memory using the virtual addresses. The virus processing hardware accelerator scans the data segment for viruses by executing pattern comparisons against the data segment. The virus processing hardware accelerator returns a result of the scanning to the general purpose processor by writing the result to the system memory. The general purpose processor may scan the data segment for viruses of a first type in parallel or serially with virus processing hardware accelerator scanning for viruses of a second type.
Owner:FORTINET
Method, device and storage medium for processing virus
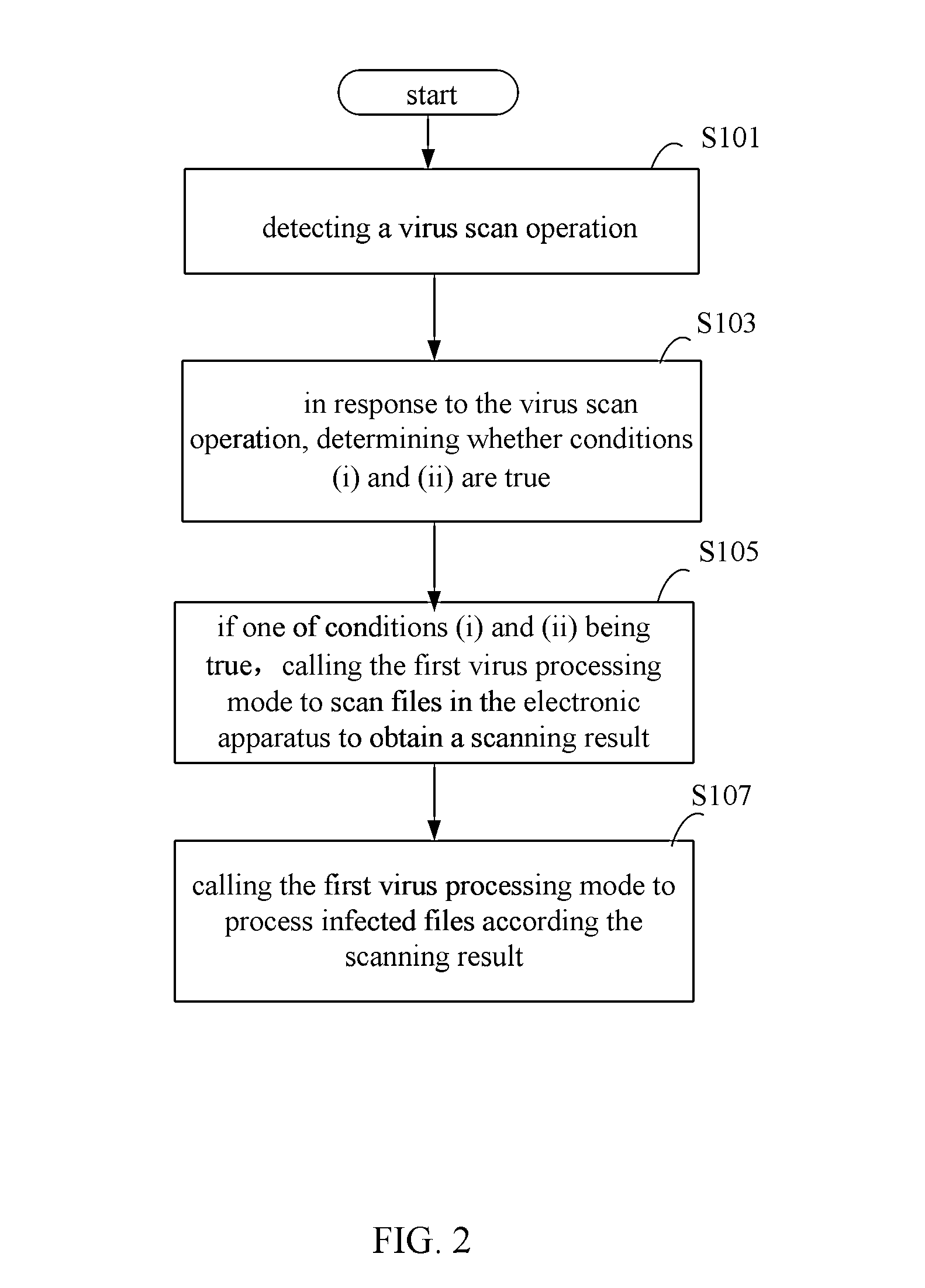
ActiveUS20140366138A1Avoid spreadingMemory loss protectionError detection/correctionComputer scienceVirus processing
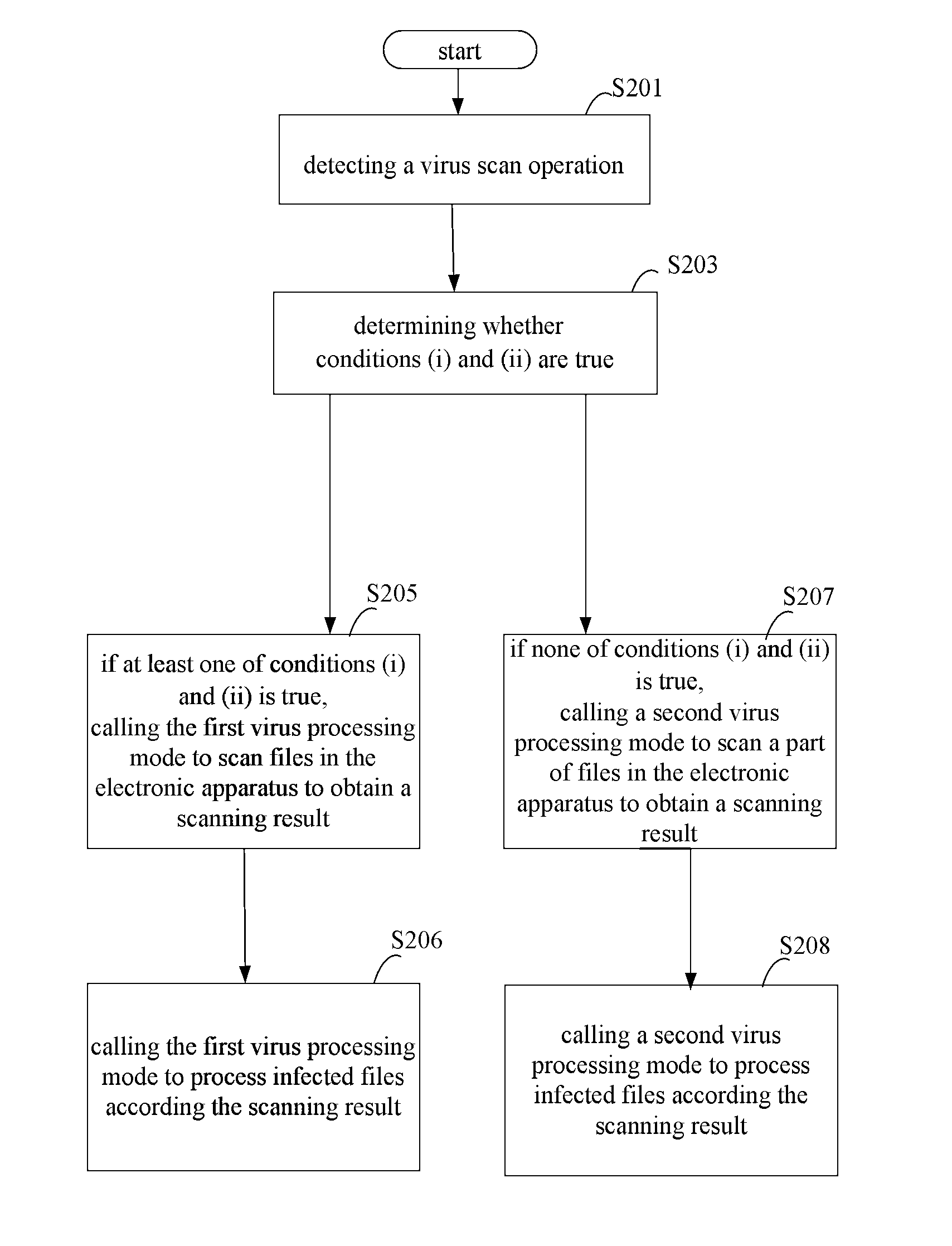
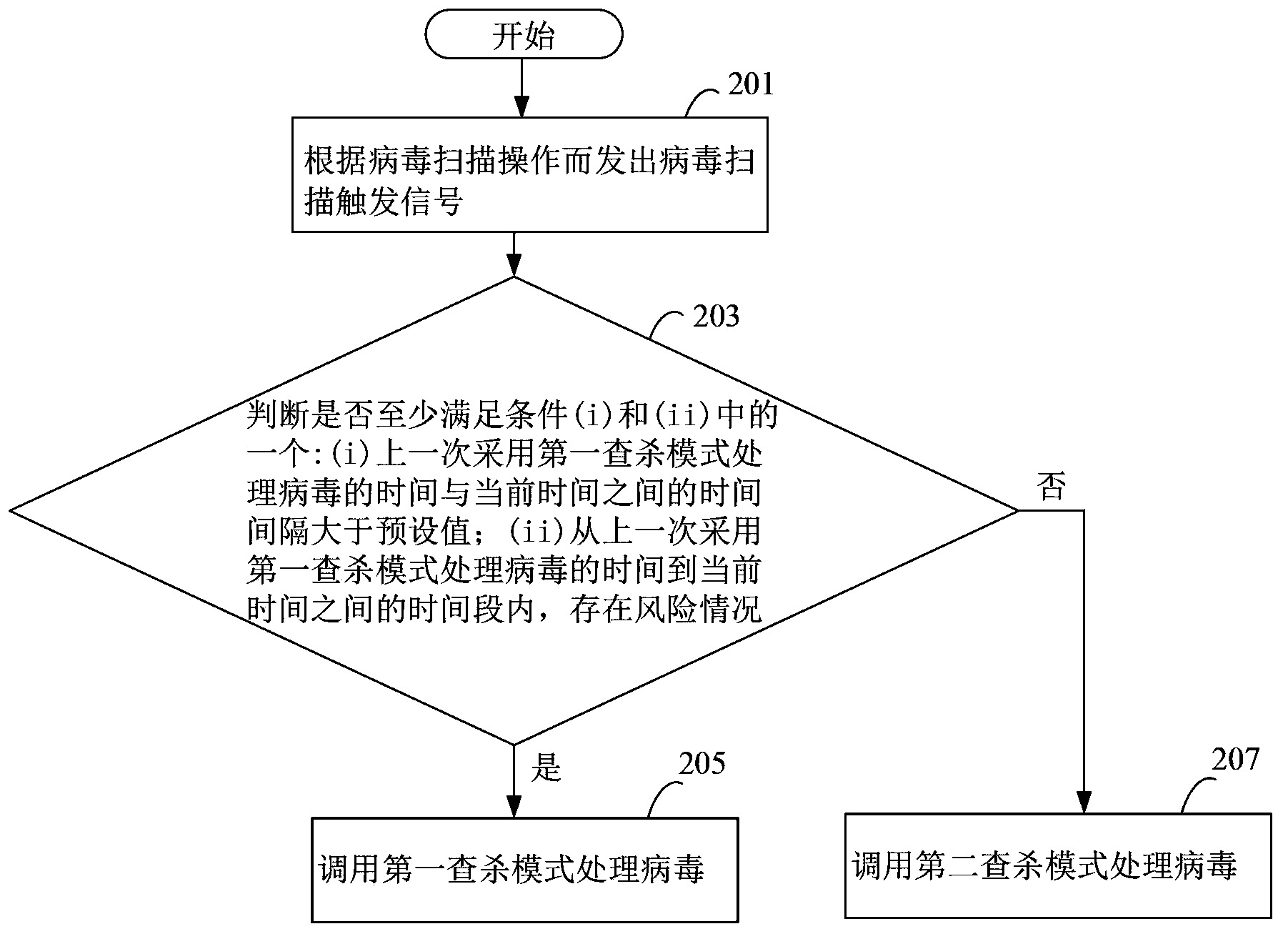
The present disclosure relates to a method, a device and a storage medium for processing virus which can automatically distinguish which of processing mode is best for the current status of the electronic apparatus. The method includes: detecting a virus scan operation; in response to the virus scan operation, determining whether conditions (i) and (ii) are true, wherein the condition (i) is true when a time interval between a last time of processing virus using a first virus processing mode and the current time is larger than a preset interval, the condition (ii) is true when at least one of risk situations exist during a time period between the last time of processing virus using the first virus processing mode and the current time; if one of conditions (i) and (ii) being true, calling the first virus processing mode to scan files in the electronic apparatus.
Owner:TENCENT TECH (SHENZHEN) CO LTD
Method, device and system for processing computer virus
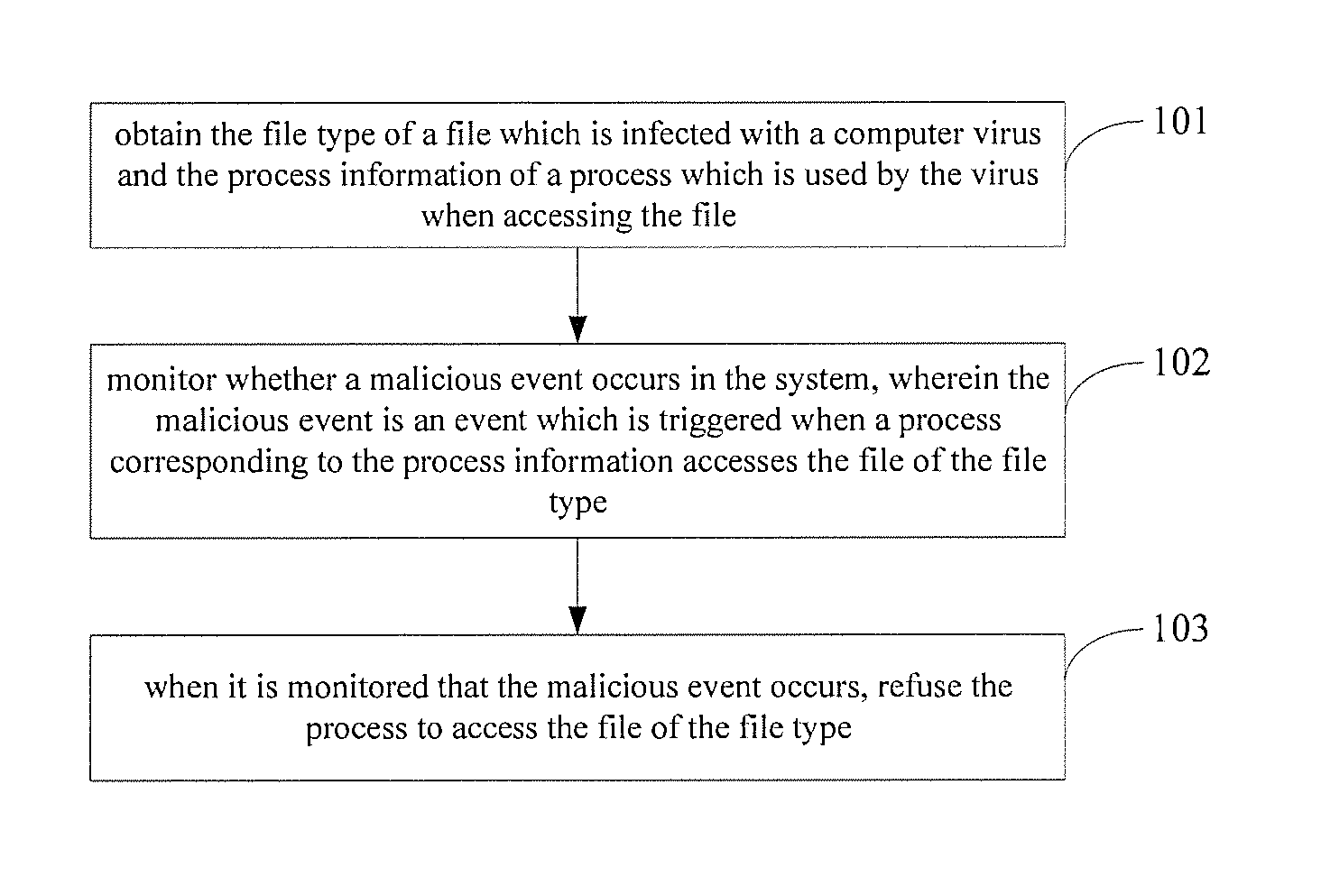
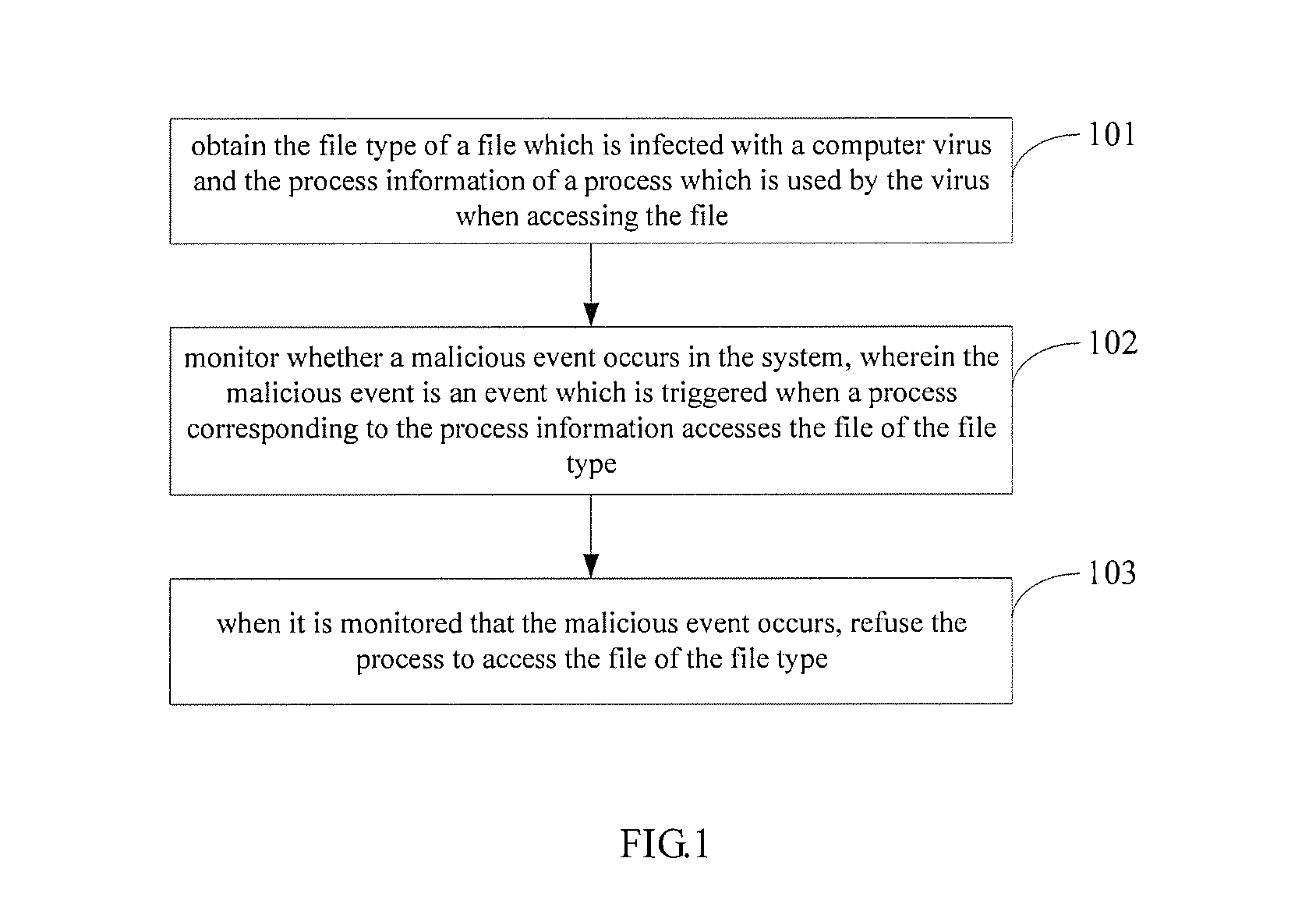
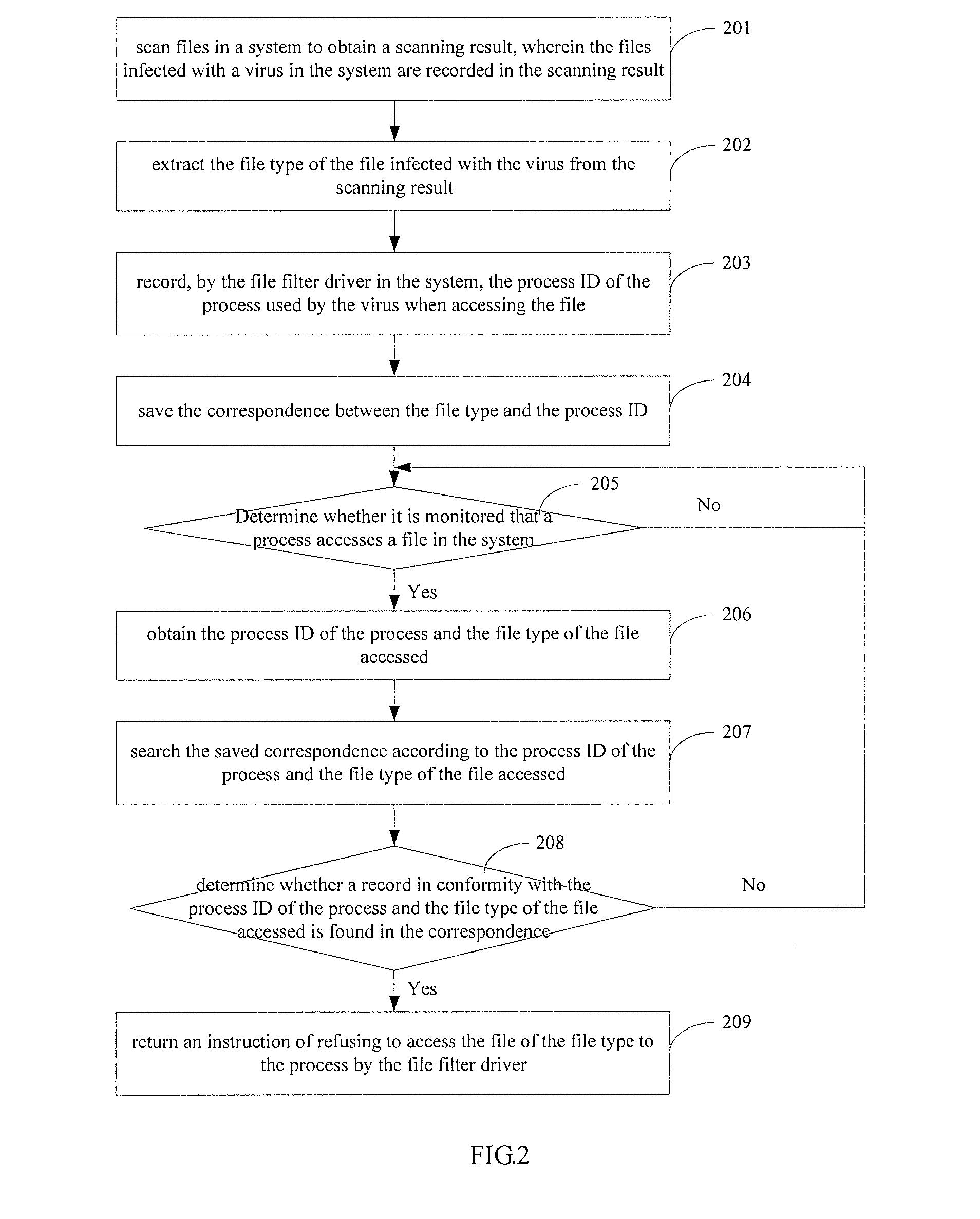
ActiveUS20140123290A1Improve securityHinders its propagationMemory loss protectionError detection/correctionSoftware engineeringProcess information
A method, an apparatus and a system for processing a computer virus. The method comprises: obtaining the file type of a file which is infected with a computer virus and the process information of a process which is used by the virus when accessing the file; monitoring whether a malicious event occurs in s system, wherein the malicious event is an event which is triggered when the process corresponding to the process information accesses the file of the file type; and refusing the process to access the file of the file type when it is monitored that the malicious event occurs.
Owner:BEIJING QIHOO TECH CO LTD
Remote antivirus method
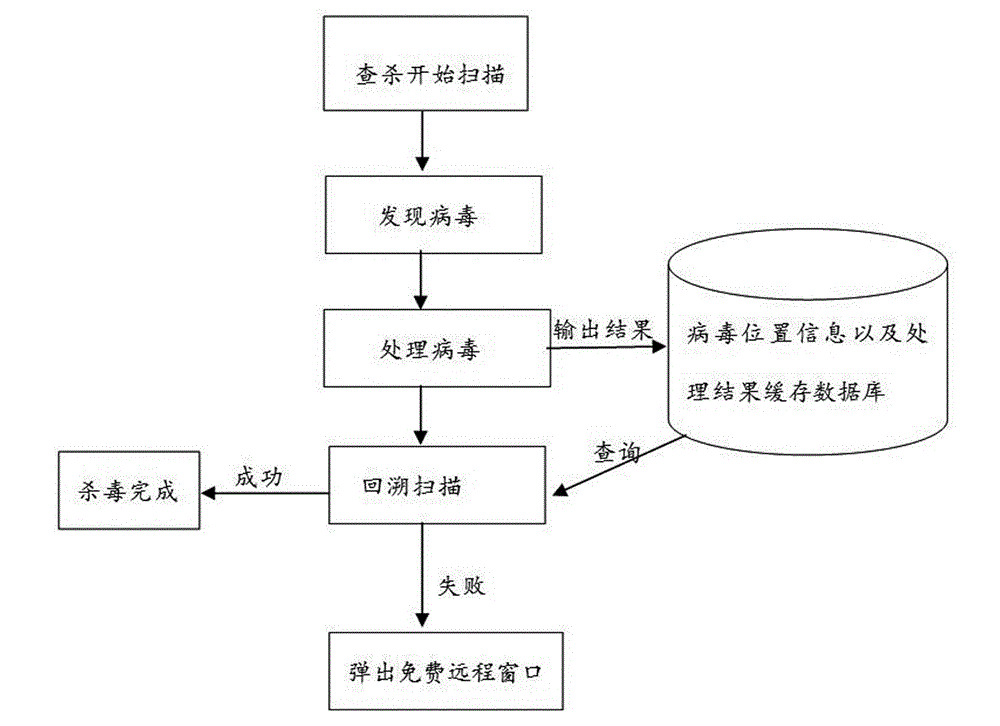
ActiveCN102945350ADelete make sureImprove antivirus effectPlatform integrity maintainanceTransmissionSecurity softwarePersonal computer
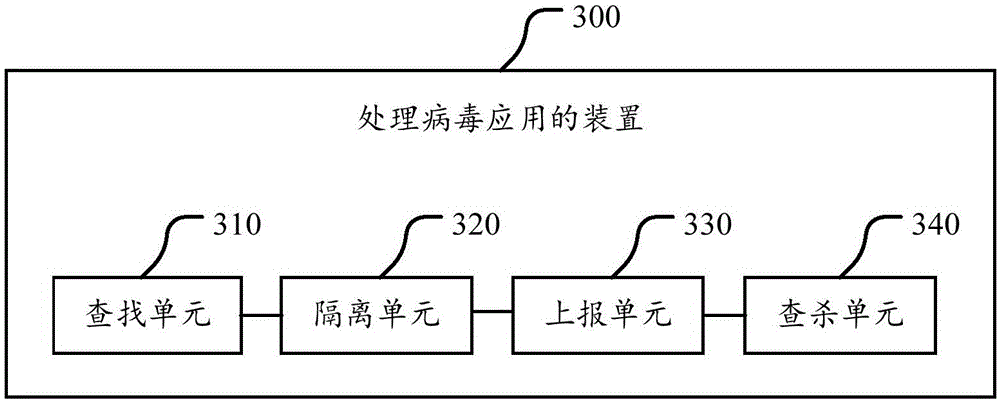
The invention provides a remote antivirus method, which comprises the following steps: processing virus after finding out the virus, and outputting virus behavior information and processed results to a cache database to establish an antivirus record; after finishing said virus processing operation, calling a scanning engine to perform scanning antivirus (backtracking antivirus) on the virus again; after performing the backtracking antivirus, judging antivirus results; reporting to a user that the antivirus is completed and finishing the operation if the virus is thoroughly cleaned; and triggering a remote help module so that a security software engineer performs manual antivirus in a personal computer by the remote help module if the virus is unable to be thoroughly cleaned. Compared with a traditional antivirus mechanism simply relying on software, the method provided by the invention is characterized by directly using professional knowledge of the professional security engineer to manually kill the virus in the personal computer and greatly enhancing the capability of the antivirus software to kill the virus and protect the personal computer.
Owner:ZHUHAI BAOQU TECH CO LTD
Virus processing method and device capable of aiming at file package
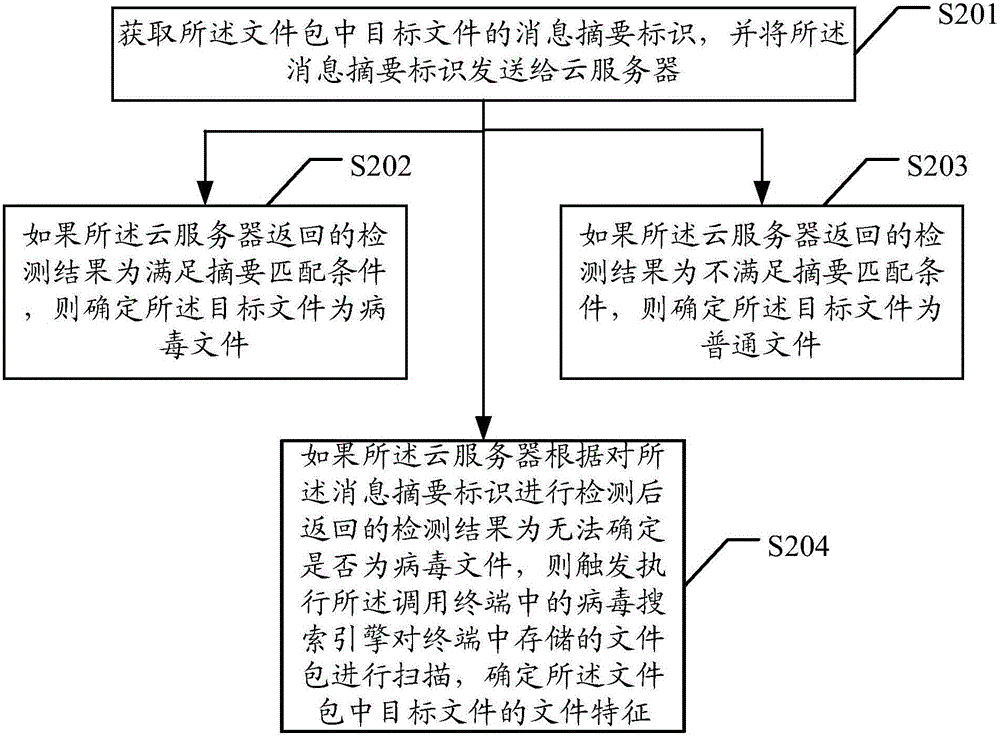
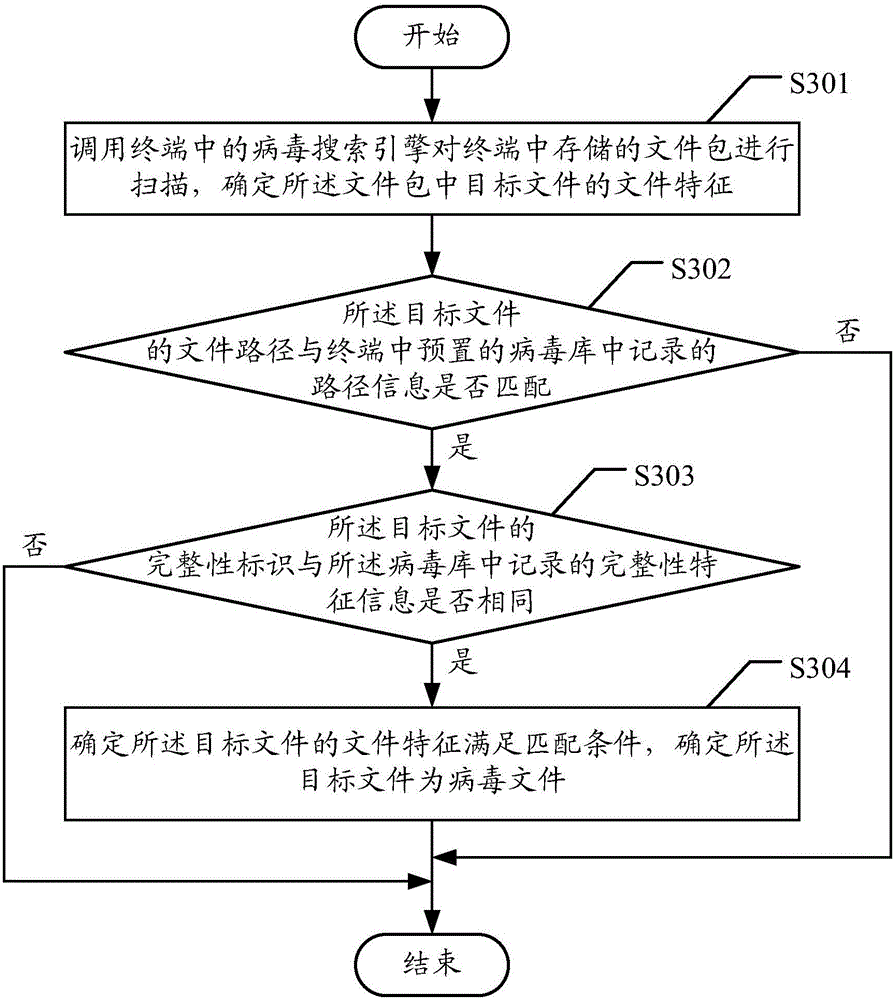
ActiveCN106709341AImprove the kill rateReduce false positivesPlatform integrity maintainanceFalse alarmPackage store
The embodiment of the invention discloses a virus processing method and device capable of aiming at a file package. The method comprises the following steps that: calling a virus search engine in a terminal to scan the file package stored in the terminal, and determining the file characteristics of a target file in the file package, wherein the file characteristics at least comprise the file path of the target file and / or the integrity identification of the target file; according to the characteristic information in a preset virus reservoir in the terminal, carrying out detection comparison on the determined file path and / or integrity identification, and when a detection comparison result shows that the file characteristics meet a matching condition, determining the target file as a virus file; and according to a virus checking and killing rule, carrying out checking and killing processing on the virus file. When the method is adopted, the virus checking and killing success rate of the file package can be conveniently and quickly improved, a variant sample checking and killing rate is improved, and false alarms are reduced.
Owner:TENCENT TECH (SHENZHEN) CO LTD
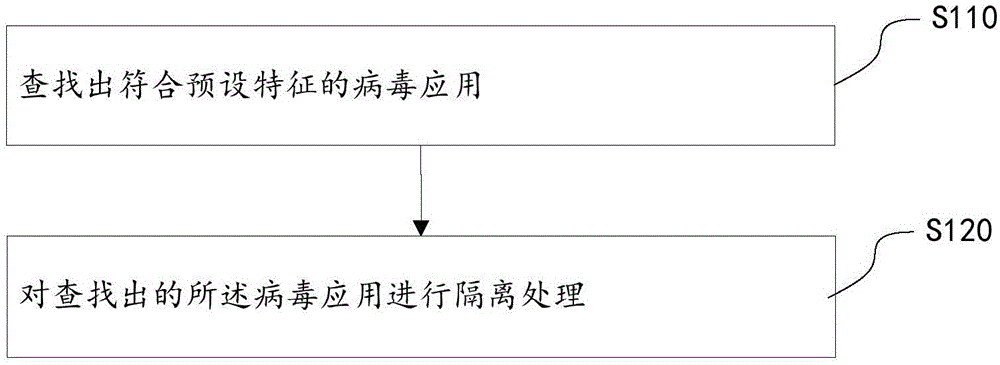
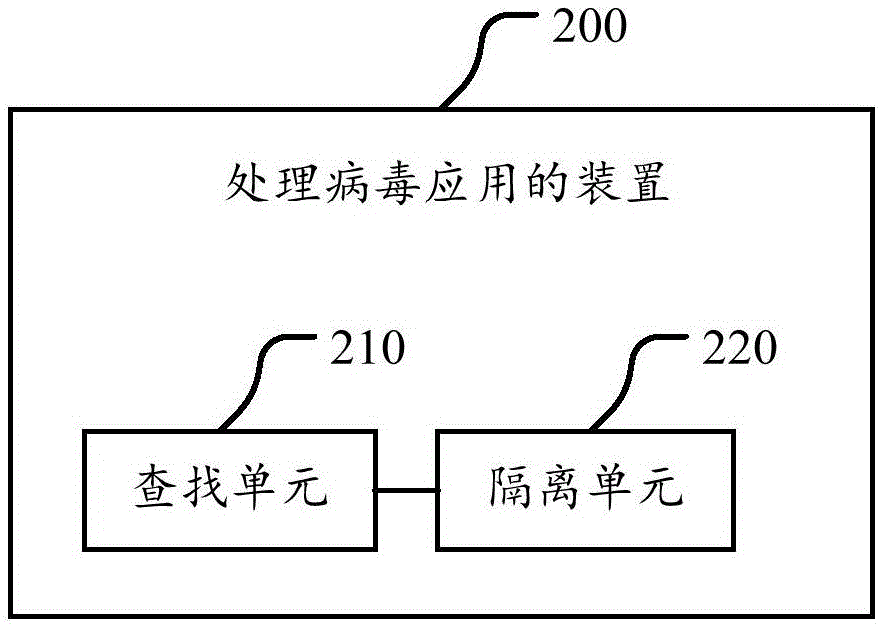
Method, device and mobile terminal for processing virus applications
InactiveCN105095754AStop runningAvoid harmPlatform integrity maintainanceComputer scienceVirus processing
The invention discloses a method, device and mobile terminal for processing virus applications. The method comprises the following steps: searching for virus applications which conform to a preset feature; and performing isolation processing on the searched virus applications. According to the technical scheme of the invention, certain stubborn virus applications are isolated when the virus applications cannot be deleted, so that the virus applications are prevented from running; the harms of the virus applications to a user are avoided; and a rapid virus processing scheme and a safer use environment are provided for the user.
Owner:BEIJING QIHOO TECH CO LTD +1
Computer system for ensuring information security and control method thereof
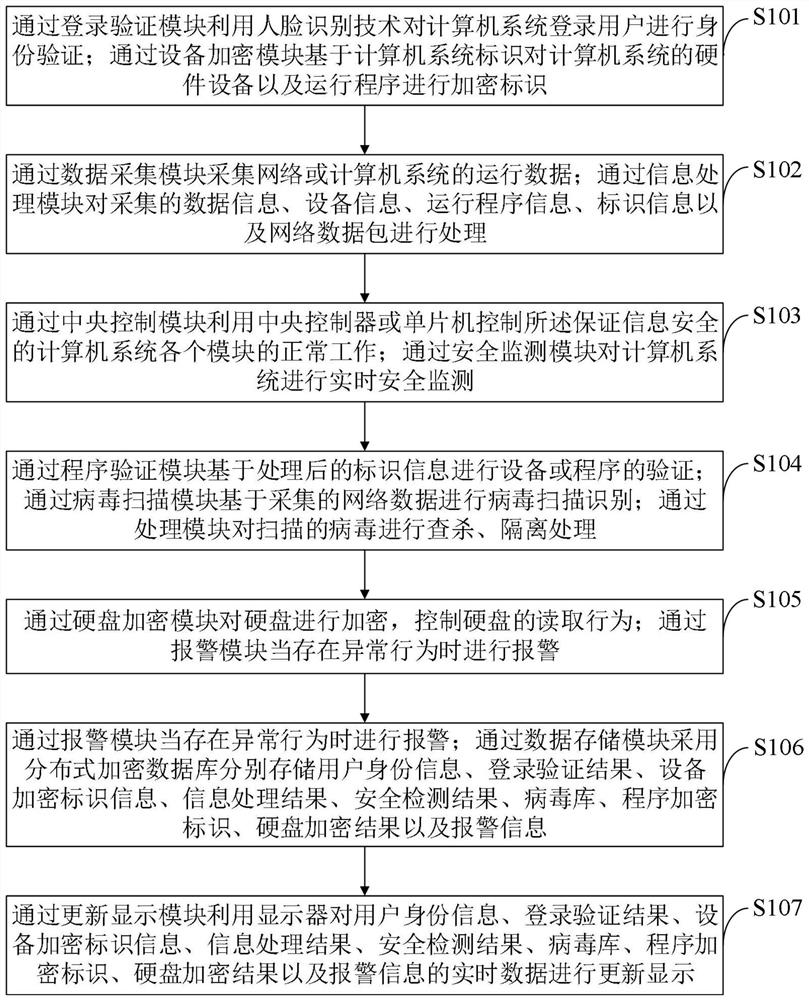
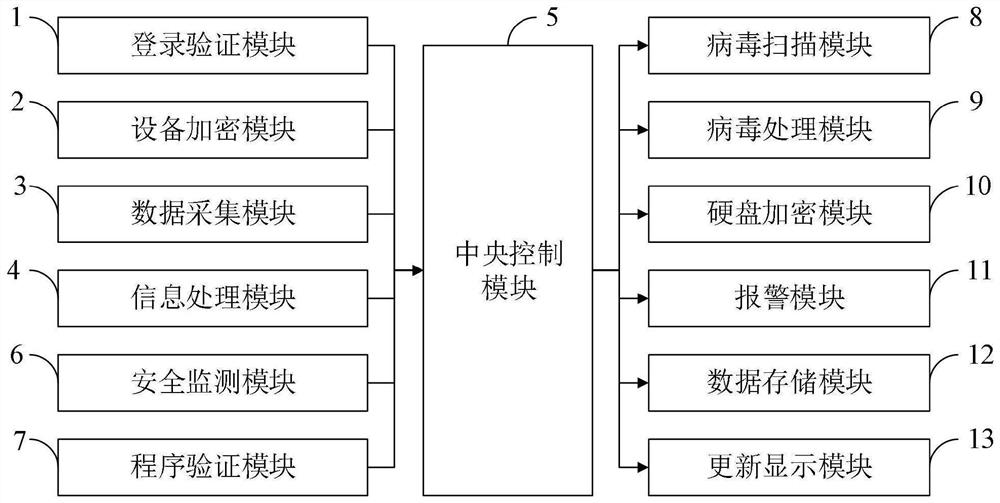
ActiveCN112487383AEnsure information securityGuarantee the safety of useCharacter and pattern recognitionDigital data protectionInformation processingData acquisition
The invention belongs to the technical field of computer security, and discloses a computer system for ensuring information security and a control method thereof. The computer system capable of ensuring information security comprises a login verification module, an equipment encryption module, a data acquisition module, an information processing module, a central control module, a security monitoring module, a program verification module, a virus scanning module, a virus processing module, a hard disk encryption module, an alarm module, a data storage module and an update display module. According to the invention, the use security of the computer can be effectively ensured, data leakage and network virus invasion are prevented, and the information security of a user is effectively ensured. The computer system capable of ensuring the information security can protect the computer data security, effectively protect viruses and ensure the hard disk data security; by performing autonomousencryption verification on the hard disk, the tedious operation of manual password input is avoided, the encryption verification efficiency is improved, and the data security of the hard disk can alsobe ensured.
Owner:CHONGQING UNIV OF EDUCATION
Virus processing method and device
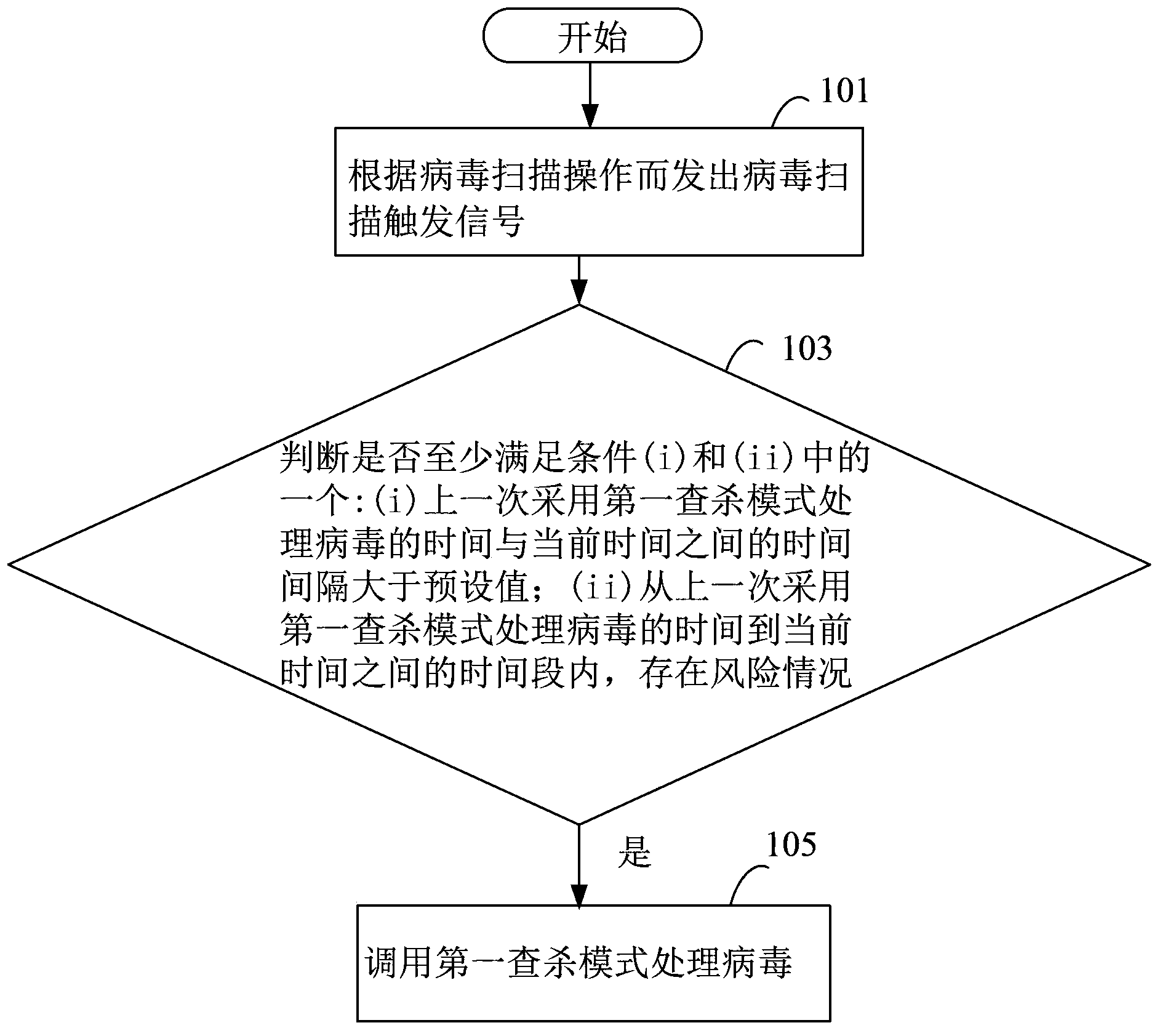
ActiveCN104239790AStop the spreadFast automatic resolutionPlatform integrity maintainanceTime segmentKilled process
The invention discloses a virus processing method and device, and belongs to the safety field. The virus processing method comprises the steps of: sending a virus scanning trigger signal based on a virus scanning operation; receiving the virus scanning trigger signal and judging whether at least one of conditions (i) and (ii) below is satisfied: (i) the time interval between the time taken for processing the virus by adopting a first searching and killing mode in the last time and the current time is greater than a preset value, and (ii) a risk condition exists within the time bucket between the time taken for processing the virus by adopting the first searching and killing mode in the last time and the current time; if at least one of the conditions (i) and (ii) is satisfied, calling the first searching and killing mode to process the virus. Through automatic selection of the virus processing mode, which searching and killing mode is most suitable for the current condition of an electronic device can be recognized rapidly and automatically in the virus searching and killing process, and the viral transmission is prevented in time.
Owner:TENCENT TECH (SHENZHEN) CO LTD
Method for preparing chimeric antigen receptor T cells through serum-free culture
ActiveCN110499291AEfficient preparationGenetic material ingredientsMammal material medical ingredientsAntigen receptorSerum ige
The invention provides a method for preparing chimeric antigen receptor T cells through serum-free culture. Specifically, the method comprises the steps that (a) peripheral blood mononuclear cells (PBMCs) are provided; (b) the PBMCs are subjected to negative sorting treatment to obtain sorted PBMCs; (c) the sorted PBMCs are activated to obtain activated T cells; (d) the activated T cells are subjected to gene transfection through a virus vector expressing a chimeric antigen receptor, and thus transfected T cells are obtained; (e) the transfected T cells are subjected to virus removal treatment, and thus T cells with viruses removed are obtained; and (f) the T cells with viruses removed are subjected to multiplication culture, and thus the chimeric antigen receptor T cells are obtained. Through the culture process, the culture success rate of the chimeric antigen receptor T cells is greatly increased, and the safety and effectiveness of the chimeric antigen receptor T cells are greatlyimproved.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
Anti-computer virus mobile storage apparatus and anti-computer virus method
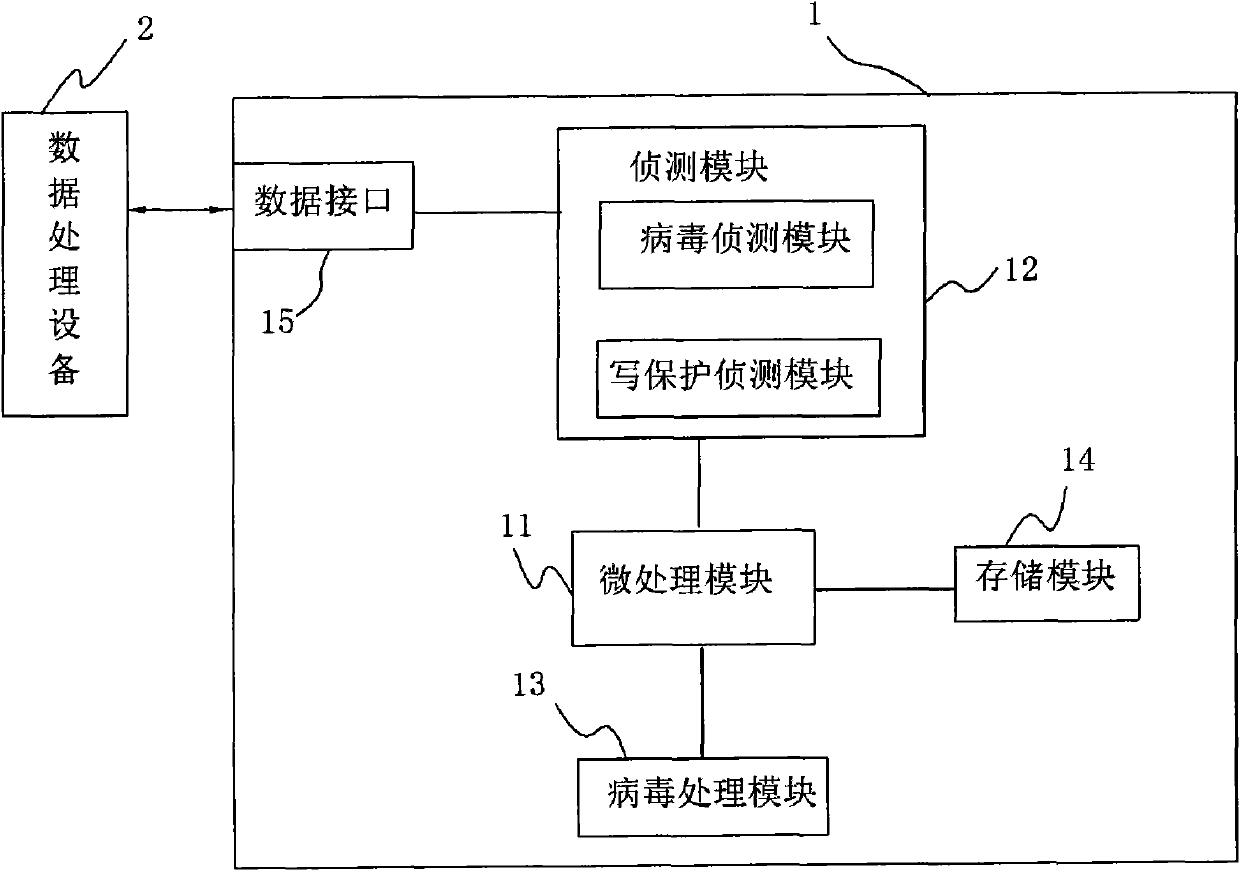
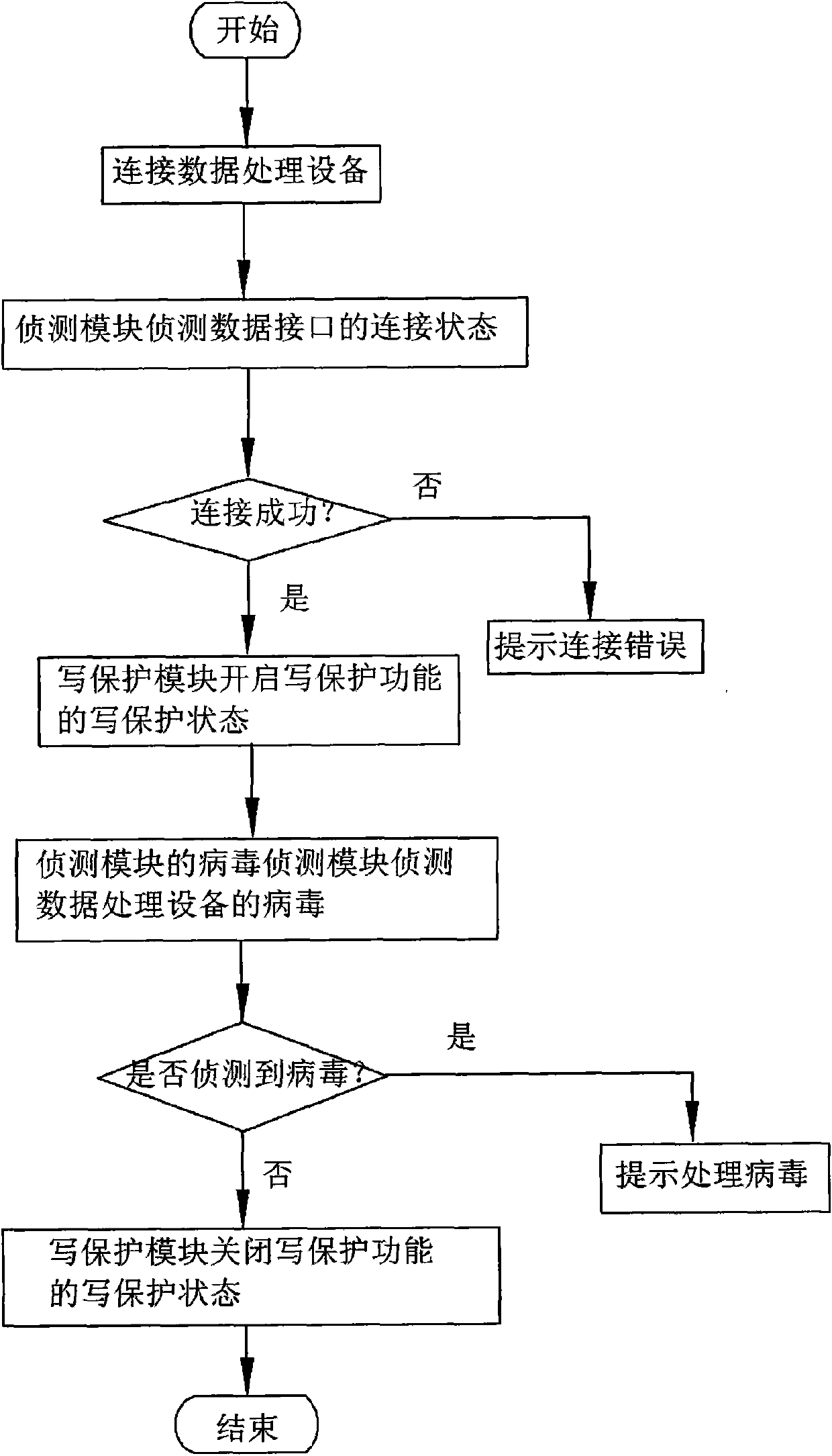
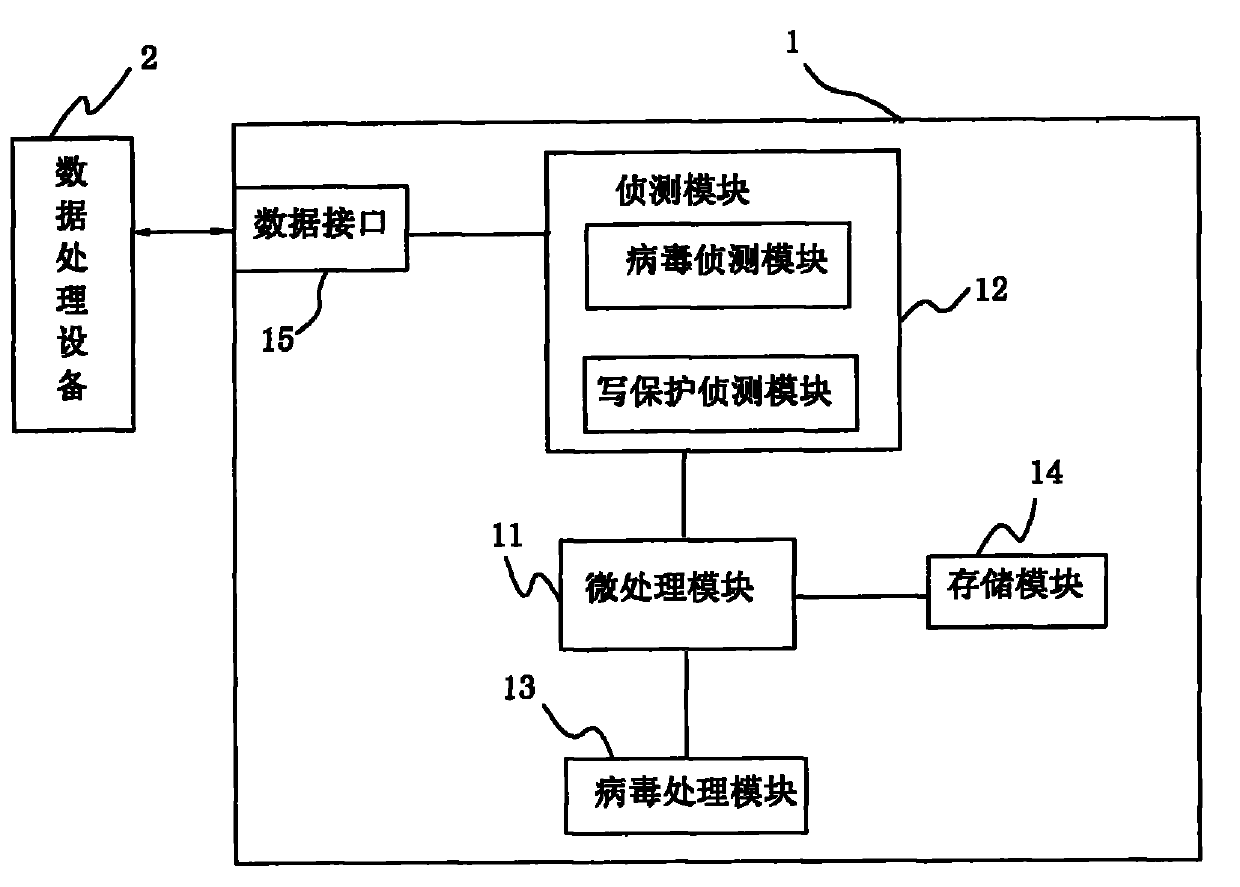
InactiveCN102023933AAvoid transmissionPrevent proliferationUnauthorized memory use protectionWrite protectionRemovable media
The invention discloses an anti-computer virus mobile storage apparatus and an anti-computer virus method. The mobile storage apparatus comprises a virus detecting module and a virus processing module. The original state of the mobile storage apparatus is set as a write-protection state through the processing module. After the mobile storage apparatus is connected with a piece of data processing equipment and safety is determined through scan programs, the virus processing module calls a write-protection module of a micro processing module to close the write-protection function, and furthermore the mobile storage apparatus can carry out data transmission with the data processing equipment. The invention adds the detecting module and the virus processing module to the mobile storage apparatus, which prevents the virus from passing through data read-write transmission of the mobile storage apparatus, raises data security of the mobile storage apparatus and avoids further virus infection.
Owner:AIGO ELECTRONICS TECH
Method for searching and killing virus of network equipment
InactiveCN1983927AImprove securityReduce attackDigital data processing detailsUser identity/authority verificationAnti virusSelf reduction
This invention is concerned with the method for checking and killing virus for network equipment includes: 1) the network equipment receives the information and calls the checking and killing virus module to processes the virus processing; 2) the checking and killing virus module sends the feedback information to the network equipment information handling module after the virus treatment for continuing disposal. The invention can use the network equipment checking and killing virus technique project on the information that sends to the terminal, reduce the attack to the terminal equipment by the virus and the maintenance the cost for the terminal, improve the reliability of the terminal; because of the equipment includes anti-virus software, and virus self-reduction, which reduces attack from the virus to the equipment, improve network safety.
Owner:HUAWEI TECH CO LTD
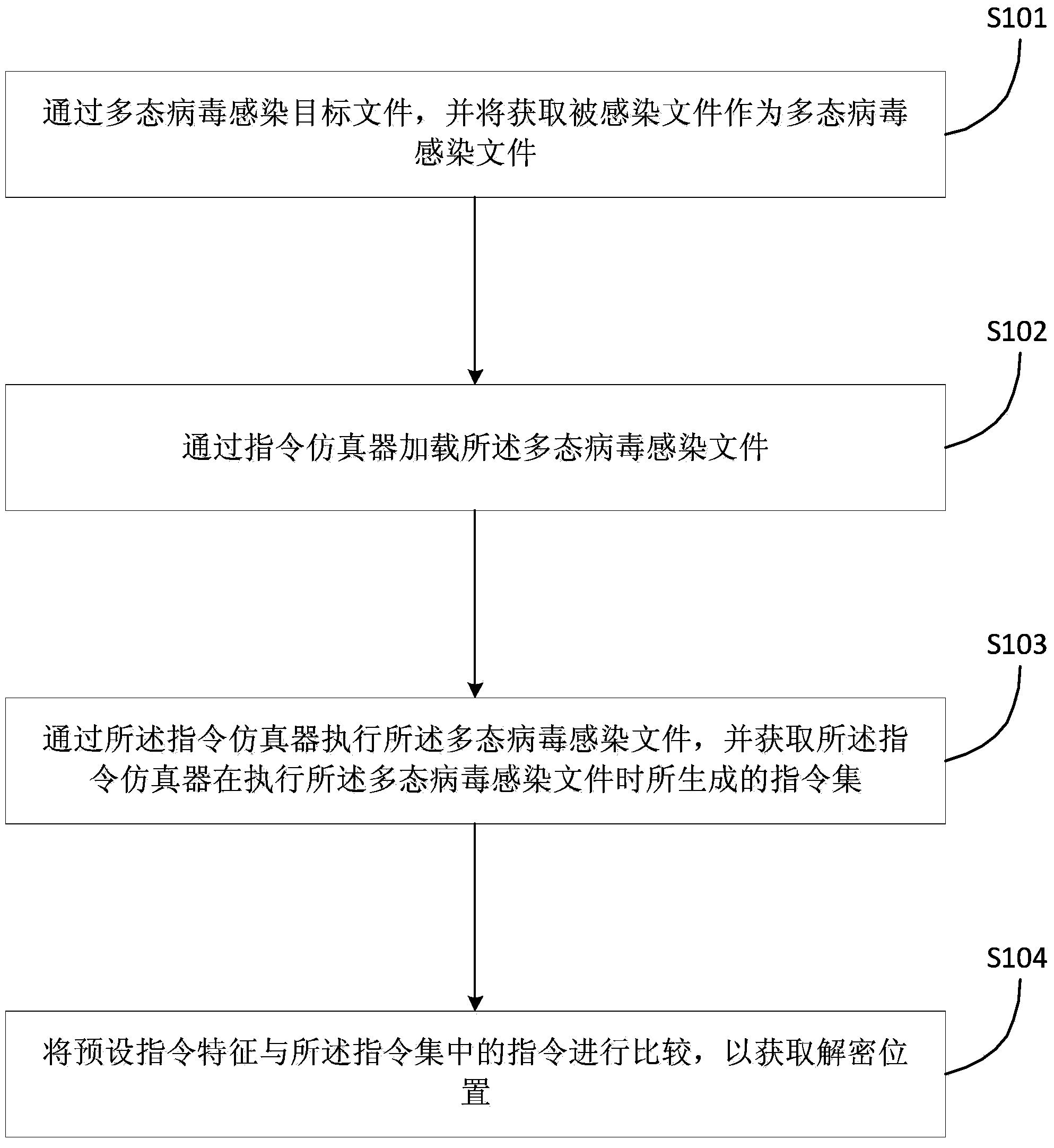
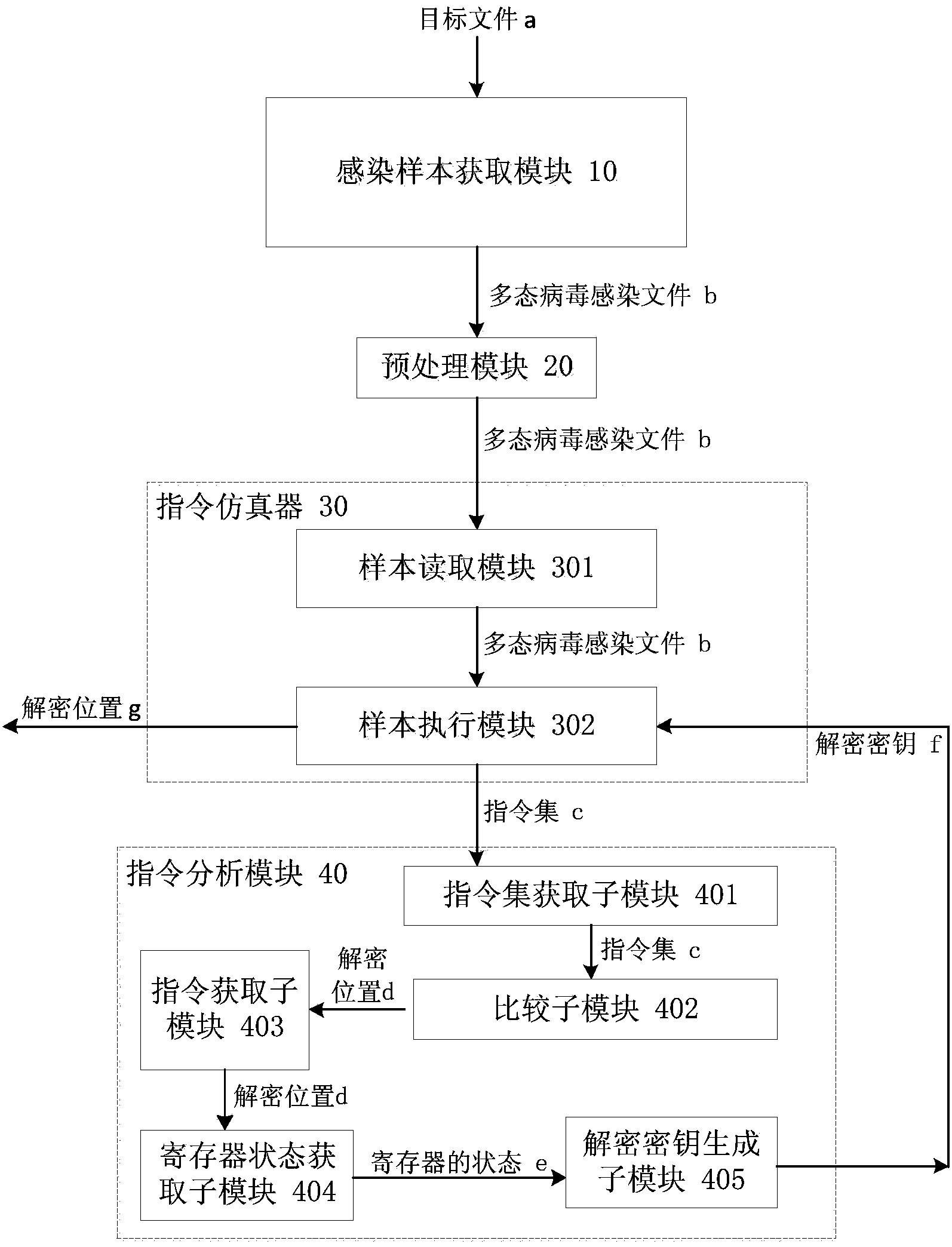
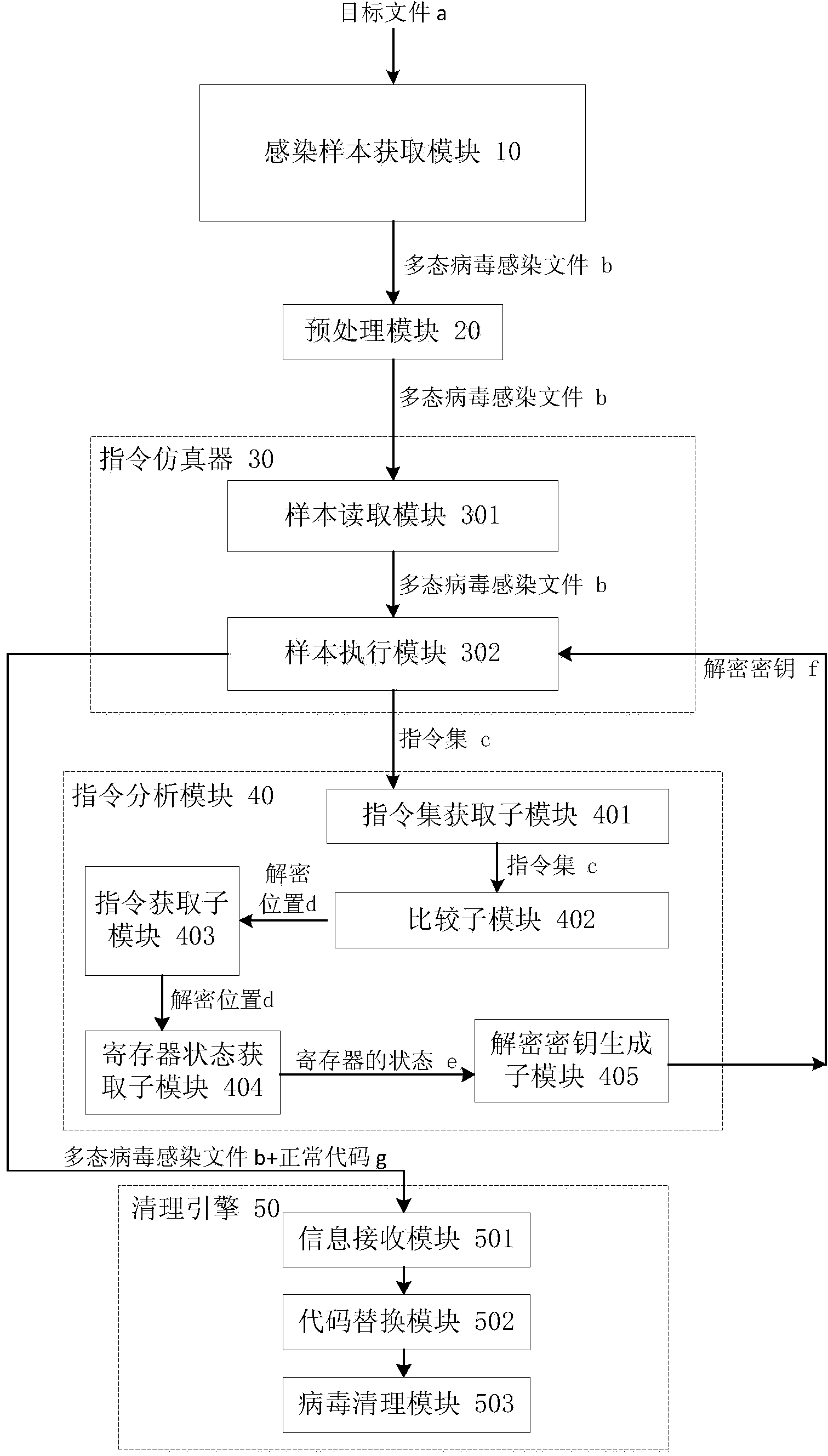
Polymorphic virus analyzing method and device and virus processing method and device
ActiveCN104077526ASimple processing methodReduce workloadPlatform integrity maintainanceInfectious virusWorkload
The invention discloses a polymorphic virus analyzing method and device and a virus processing method and device. The polymorphic virus analyzing method includes infecting a target file through polymorphic virus and utilizing the acquired infected file as a polymorphic virus infected file; loading the polymorphic virus infected file through a command simulator; utilizing the command simulator to execute the polymorphic virus infected file and acquiring a command set generated by the command simulator during execution of the polymorphic virus infected file; comparing the preset command characteristic and concentrated commands to acquire the decryption position. The polymorphic virus analyzing method simplifies the polymorphic infectious virus processing method, reduces the workload of analysis of polymorphic infectious virus, and improves the analyzing efficiency.
Owner:ZHUHAI BAOQU TECH CO LTD
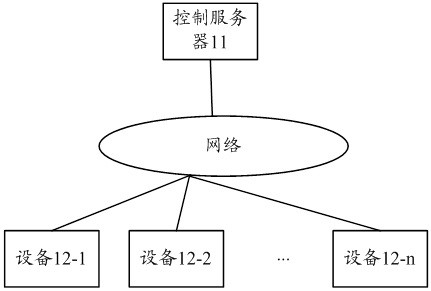
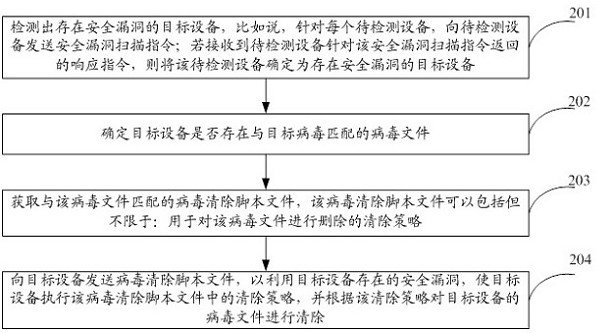
Virus processing method, device and apparatus
ActiveCN111625841AAchieve clearingSave resourcesPlatform integrity maintainanceVirus processingVulnerability scanning
The invention provides a virus processing method, device and apparatus. The method comprises the steps of sending a security vulnerability scanning instruction to a to-be-detected apparatus; if a response instruction returned by the to-be-detected apparatus for the security vulnerability scanning instruction is received, determining the to-be-detected apparatus as a target apparatus with securityvulnerabilities; determining whether a virus file matching the target virus exists in the target d or not; if so, obtaining a virus elimination script file matching the virus file; wherein the virus clearing script file comprises a clearing strategy for deleting the virus file; and sending the virus elimination script file to the target apparatus, so that the target apparatus executes an elimination strategy in the virus elimination script file by utilizing the security vulnerabilities existing in the target apparatus, and eliminates the virus file of the target apparatus according to the elimination strategy. According to the technical scheme, the virus file clearing operation is executed by the control server, and an antivirus software client does not need to be installed in the target apparatus.
Owner:HANGZHOU HIKVISION DIGITAL TECH
Preparation of virus release buffer solution and application thereof
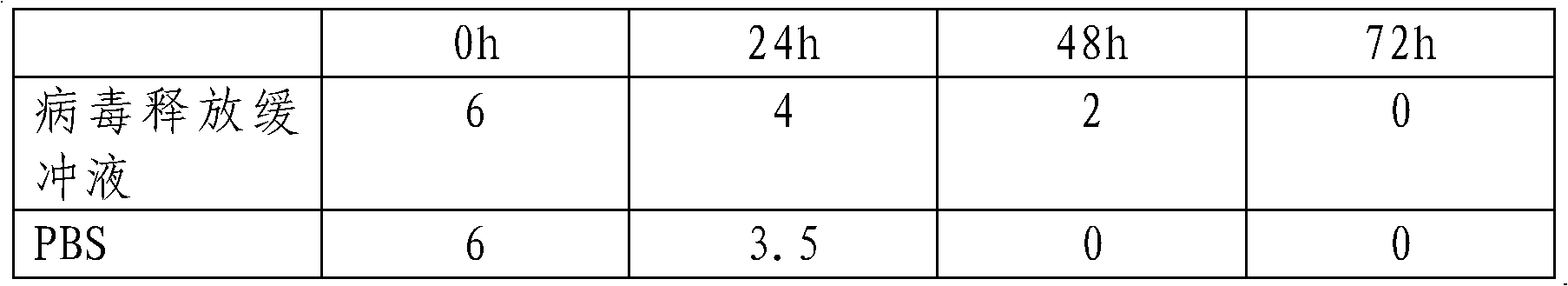
ActiveCN102533679AHigh titerImprove stabilityMicroorganism based processesRecovery/purificationVirus antigenBiomedical engineering
The invention provides a virus release buffer solution and a method for improving the blastochyle virus titer by use of the virus release buffer solution. The method comprises the following steps of: fetching the virus release buffer solution and the virus blastochyle respectively; centrifuging the virus blastochyle at an intermediate speed to separate the precipitate I and supernate I; adding the virus release buffer solution to the precipitate; mixing uniformly, and stirring for 10-30 minutes at a stirring speed of 1,000-5,000r / min at a temperature of 2-8 DEG C; centrifuging again at an intermediate speed to separate the precipitate II and supernate II; mixing the supernate II and supernate I and performing transverse tangential-flow concentration; or performing transverse tangential-flow concentration of the supernate II and supernate I respectively before mixing to obtain the concentrate of the virus blastochyle, wherein the aperture of a filter membrane for the transverse tangential-flow concentration is 10-500KDa, and the blastochyle concentration multiple is 2-10. According to the invention, the detection virus titer of the blastochyle avian virus can be improved; after virus treatment, the stability of the virus particles can be enhanced without causing influence on the virus antigen; and moreover, a great quantity of blastochyle viruses can be treated, and the treatment speed is high.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +1
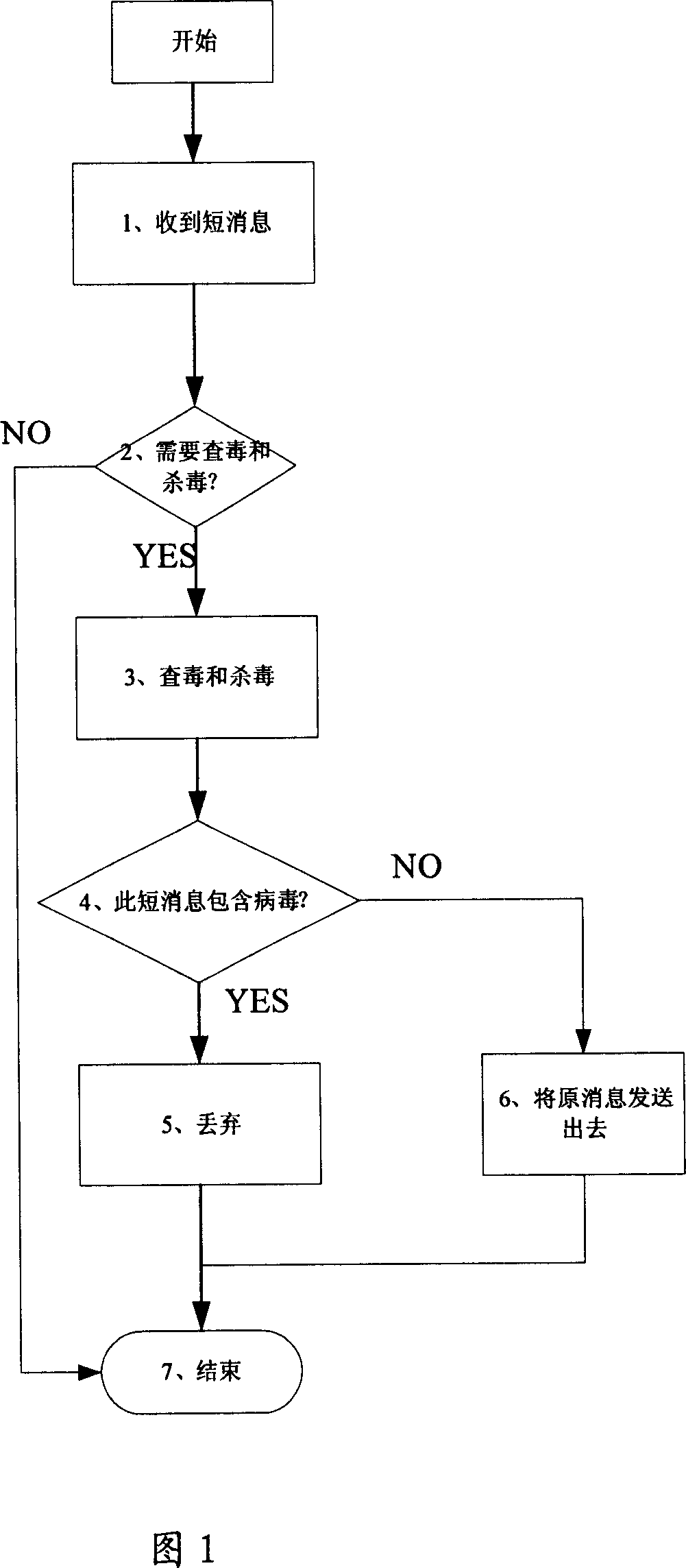
Anti-virus E-mail processing system and method
The invention discloses an anti-virus E-mail processing system and method. The anti-virus E-mail processing system comprises a virus detecting unit, a virus isolating unit and a virus processing unit, wherein the virus detecting unit is installed on a computer; the virus isolating unit is used for receiving virus detected information uploaded by the virus detecting unit and isolating the virus; the virus processing unit is used for receiving the virus detected information uploaded by the virus detecting unit and eliminating the virus. The anti-virus E-mail processing method comprises the following steps: a) detecting if the virus exists: utilizing a virus detecting module to detect if the virus exists in the E-mail when the E-mail is received by the computer; b) isolating the virus: refusing to open the E-mail and utilizing a virus isolating module of the computer to isolate the virus when the virus is detected; c) processing the virus: utilizing an antivirus program on the computer to perform anti-virus treatment on the E-mail.
Owner:DALIAN HONGYU TECH
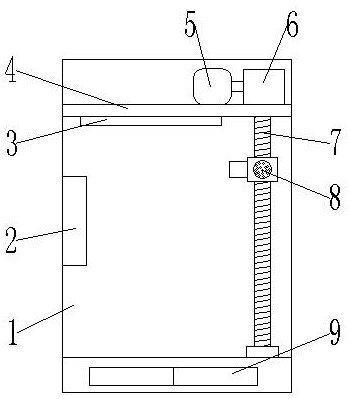
Virus treatment system for elevator car
ActiveCN112551324AImprove operational efficiencyReduce workloadLavatory sanitoryBuilding liftsElectric machineryStructural engineering
A virus treatment system for an elevator car comprises the car. A supporting plate is arranged at the top of the inner side of the elevator car, and a first motor is arranged on the upper side of thesupporting plate. An output shaft of the first motor is connected with a transmission. An output shaft of the transmission is connected with a threaded rod. A disinfection mechanism is arranged on thethreaded rod. A guide plate is arranged on the inner side of the elevator car. A guide groove is formed in the guide plate. The guide plate is located on one side of the threaded rod. One end of thedisinfection mechanism is connected with the guide groove. A sterilizing lamp is arranged on the lower side of the supporting plate. By arranging the disinfection mechanism, the elevator car can be automatically disinfected by utilizing the idle time of the elevator, so that the operation efficiency of the elevator is improved, the workload of operators is reduced, and the disinfection effect is good.
Owner:ZHONGBEI UNIV +1
Virus sample treating fluid and treating method of novel coronavirus and rapid constant-temperature reverse transcription amplification kit for detecting virus
PendingCN112195274ASmall reaction volumeLow costMicrobiological testing/measurementMicroorganism based processesPathogenic microorganismNucleic acid detection
The invention relates to a virus sample treating fluid and treating method of novel coronavirus and a rapid constant-temperature reverse transcription amplification kit for detecting the novel coronavirus, and belongs to the technical field of pathogenic microorganism in-vitro molecular detection. The virus sample treating fluid of the novel coronavirus is a guanidine hydrochloride aqueous solution with the final concentration of 0.1-1 M or a 0.1xTE buffer solution containing Tween-20 with the final mass percentage of 0.01%-0.1%. A virus sample treated with the virus treating fluid can realizesimple, convenient and rapid virus nucleic acid detection, and a result can be observed only by constant-temperature amplification and color development methods.
Owner:杭州缔蓝生物技术有限公司
Virus processing method and device, computer device and computer readable storage medium
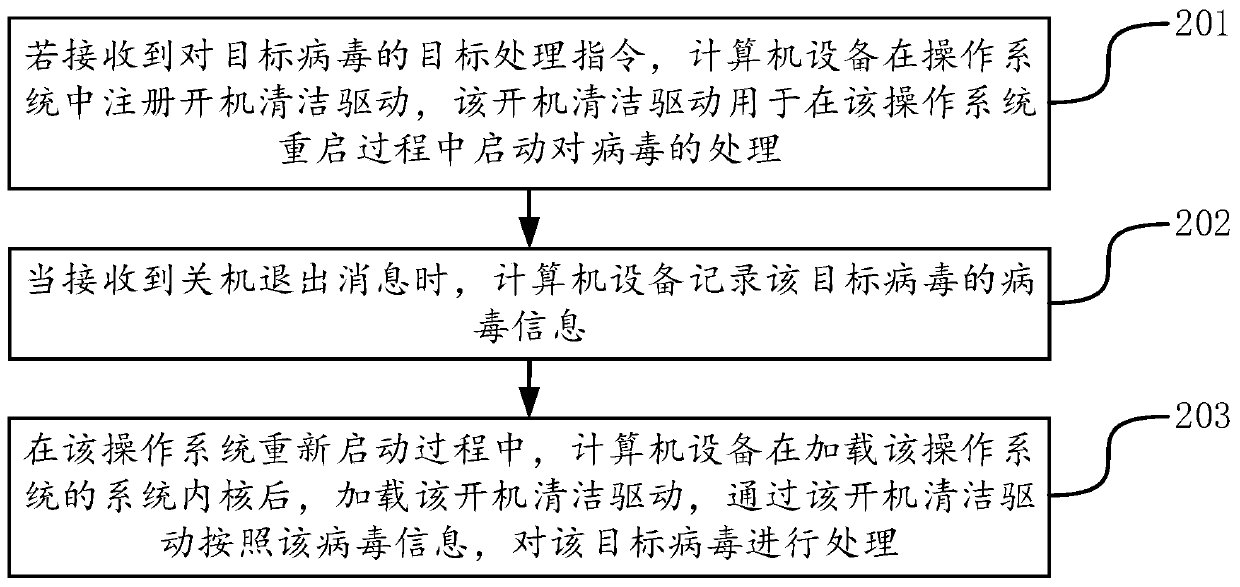
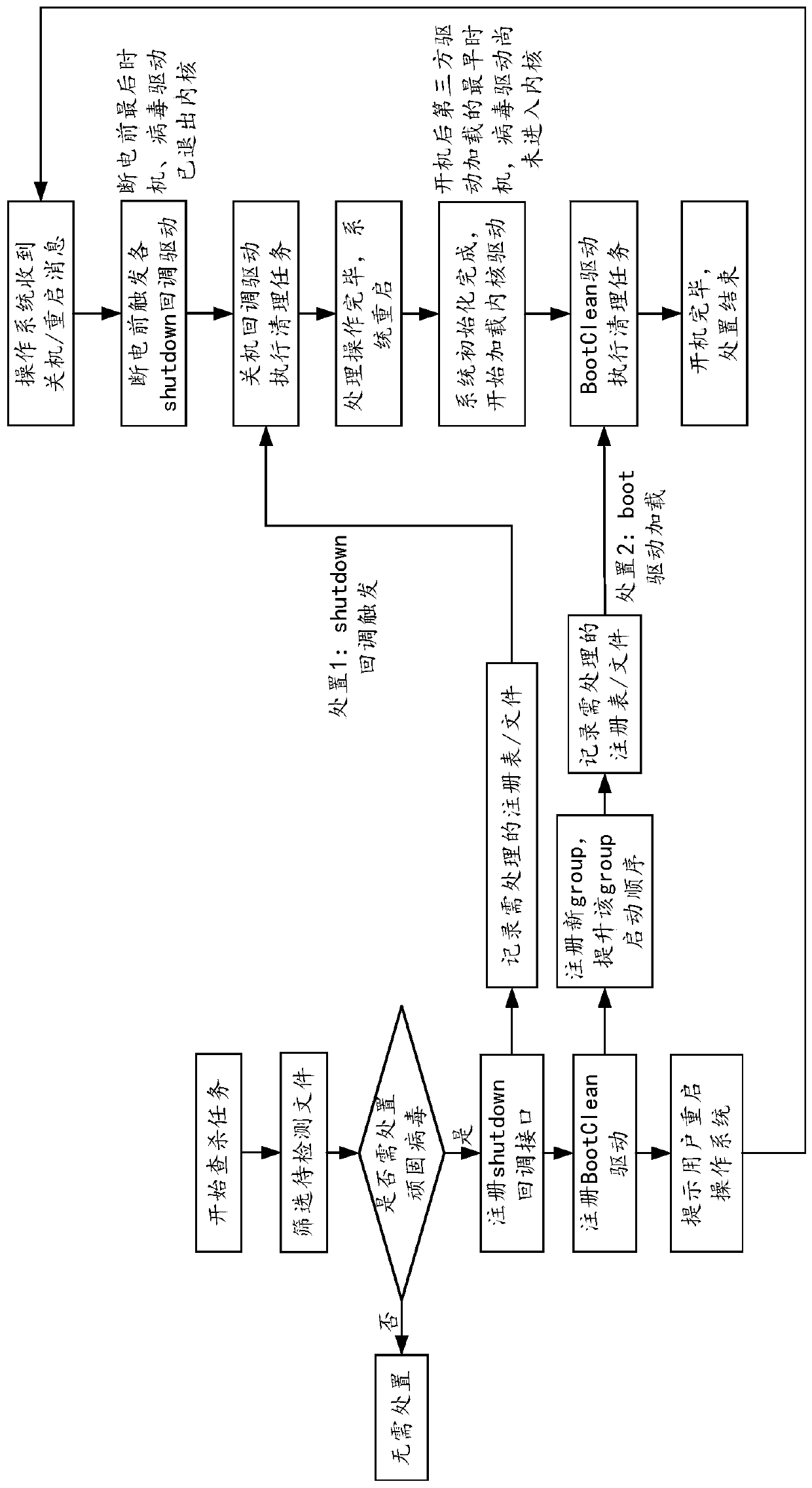
ActiveCN110851831ATimely processingTimely protectionPlatform integrity maintainanceEnergy efficient computingProcessing InstructionShut down
The invention provides a virus processing method and device, a computer device and a computer readable storage medium, and belongs to the technical field of computers. The method comprises the following steps: when a target processing instruction for a target virus is received, registering a startup cleaning drive for processing the virus in the restarting process of the operating system in the operating system, and loading the startup cleaning drive to process the target virus according to the recorded virus information of the target virus after the system kernel is completely loaded in the restarting process of the operating system. According to the method, through loading a system kernel, a startup cleaning drive registered before shutdown is loaded, and viruses are treated. Since the virus does not enter the kernel and is in the vacuum period of virus protection at the moment, deletion or blocking of virus loading is facilitated, the processing success rate is greatly increased. Inaddition, the virus can be processed in time without analyzing the hiding principle of the virus, and a computer system and computer data are protected.
Owner:TENCENT TECH (SHENZHEN) CO LTD
Computer network defense decision-making system
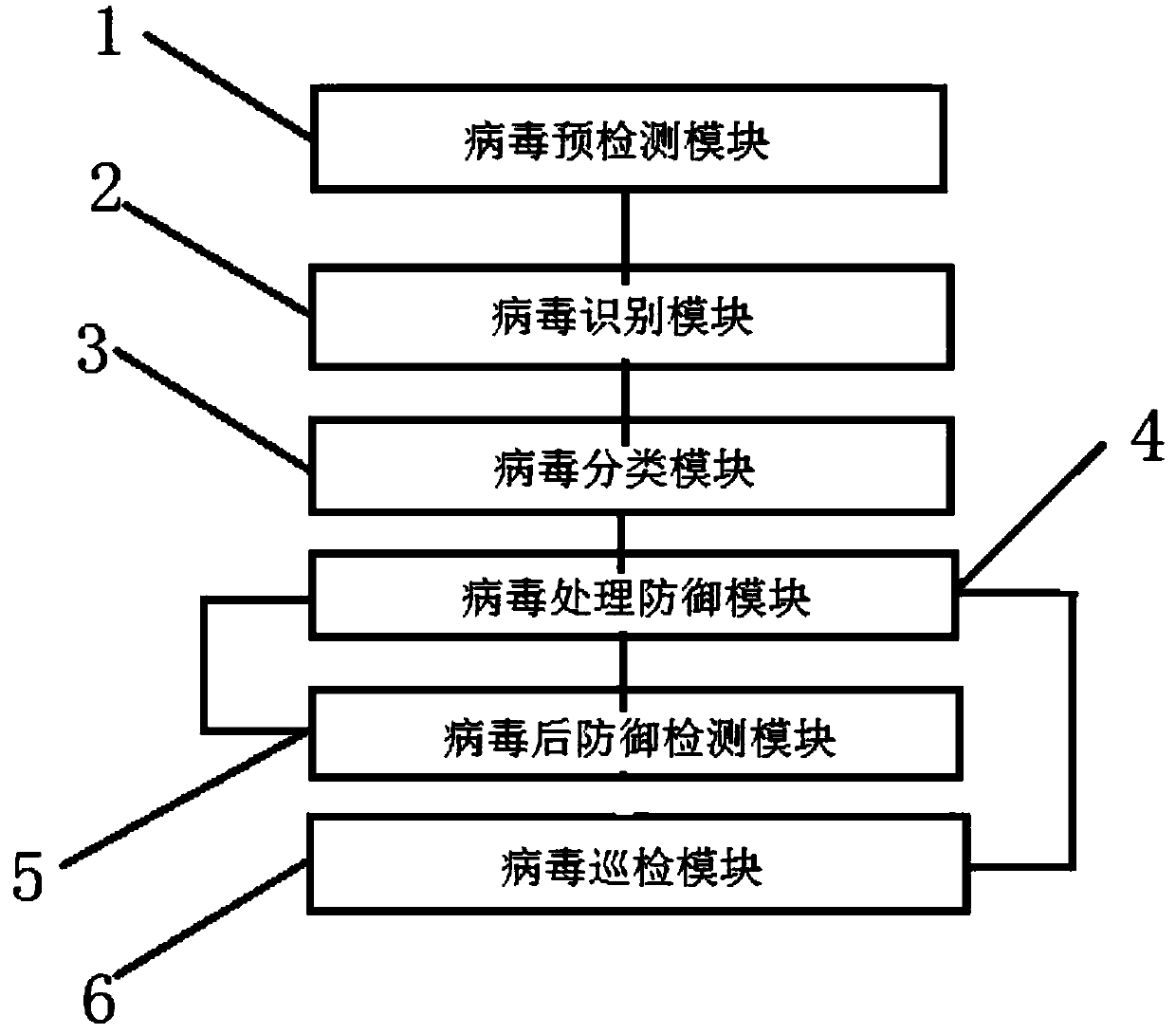
ActiveCN108540474AImprove securityClear division of laborTransmissionDecision systemVirus classification
The invention belongs to the technical field of network security and discloses a computer network defense decision-making system. A virus pre-detection module, a virus identification module, a virus classification module, a virus processing defense module and a virus post-defense detection module are connected in sequence, and a virus patrol inspection module is used for patrol monitoring. The residual virus condition is detected by the virus post-defense detection module, and the residual virus can be directly sent to the virus processing defense module to continue checking and killing. In another classification, the virus patrol inspection module exists to monitor various viruses at any time, and the virus of the same species before can be directly sent to the virus processing defense module for checking and killing. The defense decision-making system module has a clear logical hierarchy, and each module has a clear division of labor. Each module automatically monitors the virus at any time to ensure high network security and ensure permanent and complete checking and killing of the same virus.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Mask preparation method and mask prepared based on method
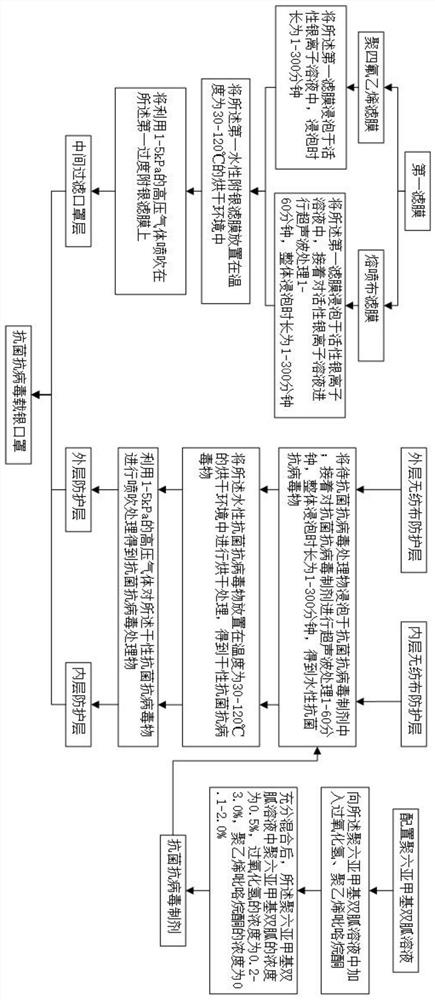
PendingCN113684681AImprove antibacterial and antiviral effectsDisinfect functionBiochemical fibre treatmentFibre typesEngineeringNonwoven fabric
The invention discloses a mask preparation method and a mask prepared based on the method. The method comprises the following steps of carrying out water-based silver attachment treatment on a first filter membrane to obtain a middle filtering mask layer; performing antibacterial and antiviral treatment on an outer non-woven fabric protective layer to obtain an outer protective layer; performing antibacterial and antiviral treatment on an inner non-woven fabric protective layer to obtain an inner protective layer; and by utilizing the outer protective layer, the middle filtering mask layer and the inner protective layer, preparing a mask body. According to the technical scheme provided by the invention, the mask has a virus killing function, and the antibacterial and antiviral effects of the mask can be improved.
Owner:HUNAN ANSON MEDICAL POLYMER MATERIALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com