Method for preparing chimeric antigen receptor T cells through serum-free culture
A chimeric antigen receptor and serum-free medium technology, applied in the biological field, can solve problems such as large differences in serum quality, introduction of mycoplasma, viruses, and hindering the development of cell therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
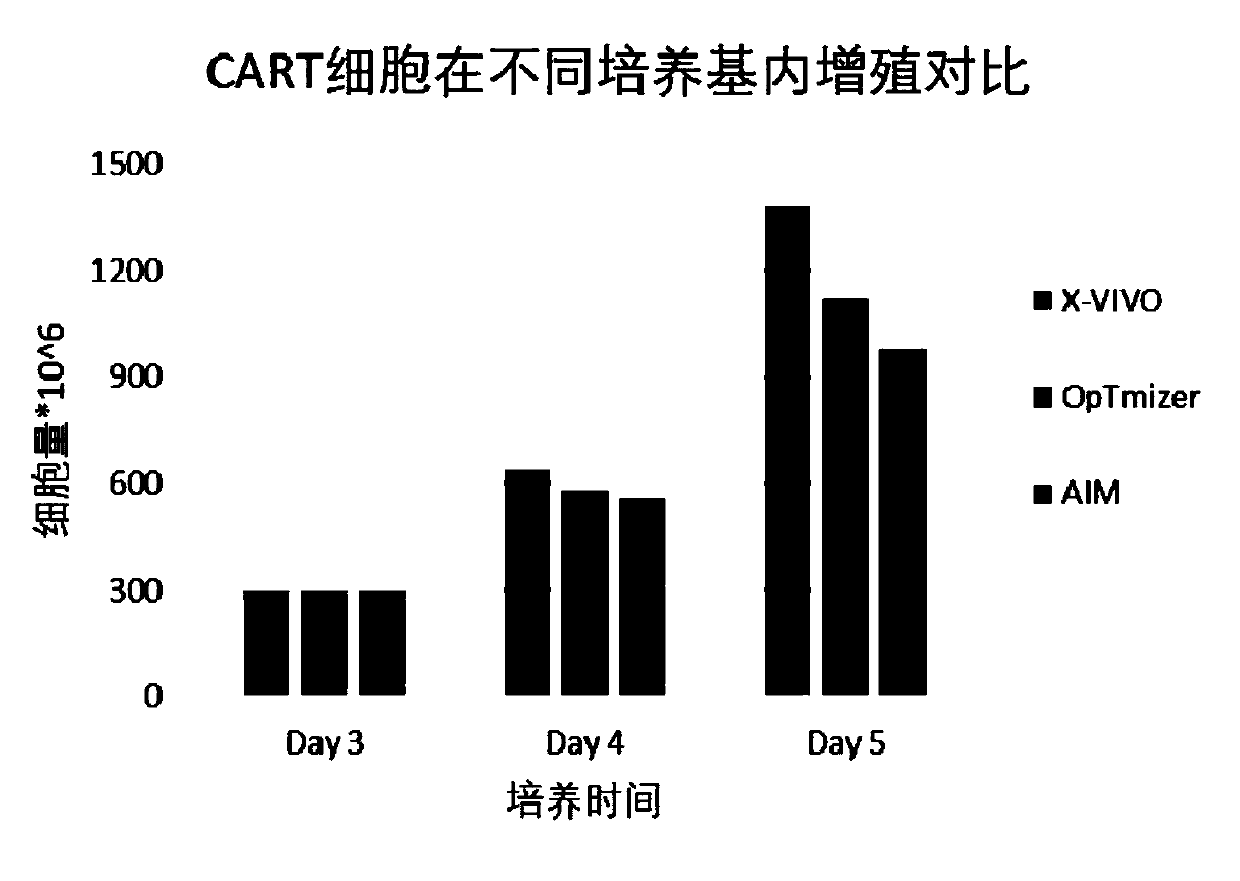
[0069] Media screening
[0070] 1.1 Frozen or fresh PBMC
[0071] Take frozen or fresh 300×10 6 -1000×10 6 Cells of PBMC were resuspended in serum-free medium.
[0072] 1.2 Sorting
[0073] PBMC were labeled with CD19 and CD14 magnetic beads and incubated for 30 minutes. Install the pipelines required for sorting on the CliniMACS as required, and perform negative sorting operation after the cell incubation to remove CD19 and CD14-labeled impurity cells, and obtain sorted cells with CD3-positive cells as the main component
[0074] 1.3 Cell activation
[0075] The sorted cells were activated with CD3 / CD28, and then 3×10 6 -6×10 6 Cell density per ml was inoculated with serum-free medium (supplemented with IL-2) containing a final concentration of 1.0% albumin, and cultured for 2 days.
[0076] 1.4 Gene transduction
[0077] After the cells were centrifuged to discard the supernatant, add the required volume of lentivirus according to MOI 2-10, resuspend in serum-free m...
Embodiment 2
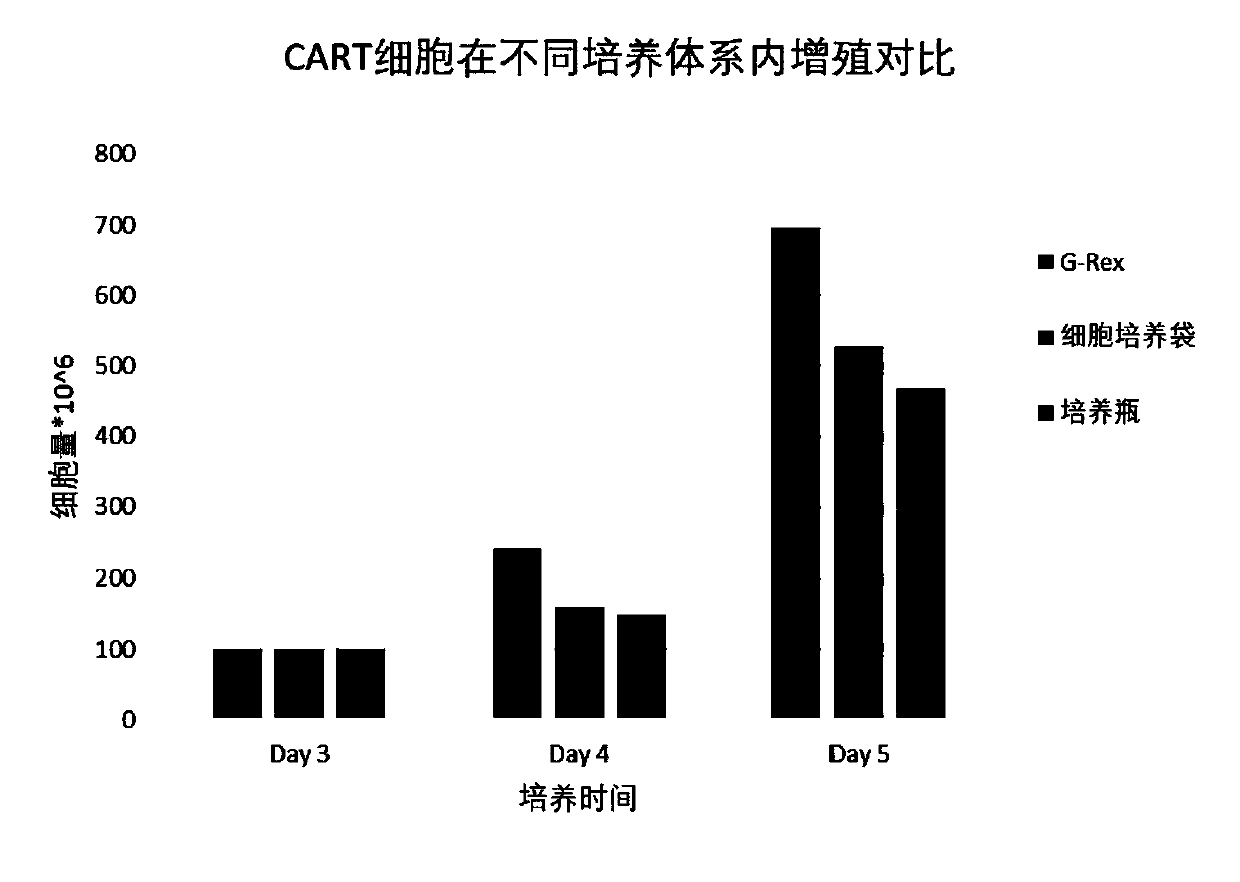
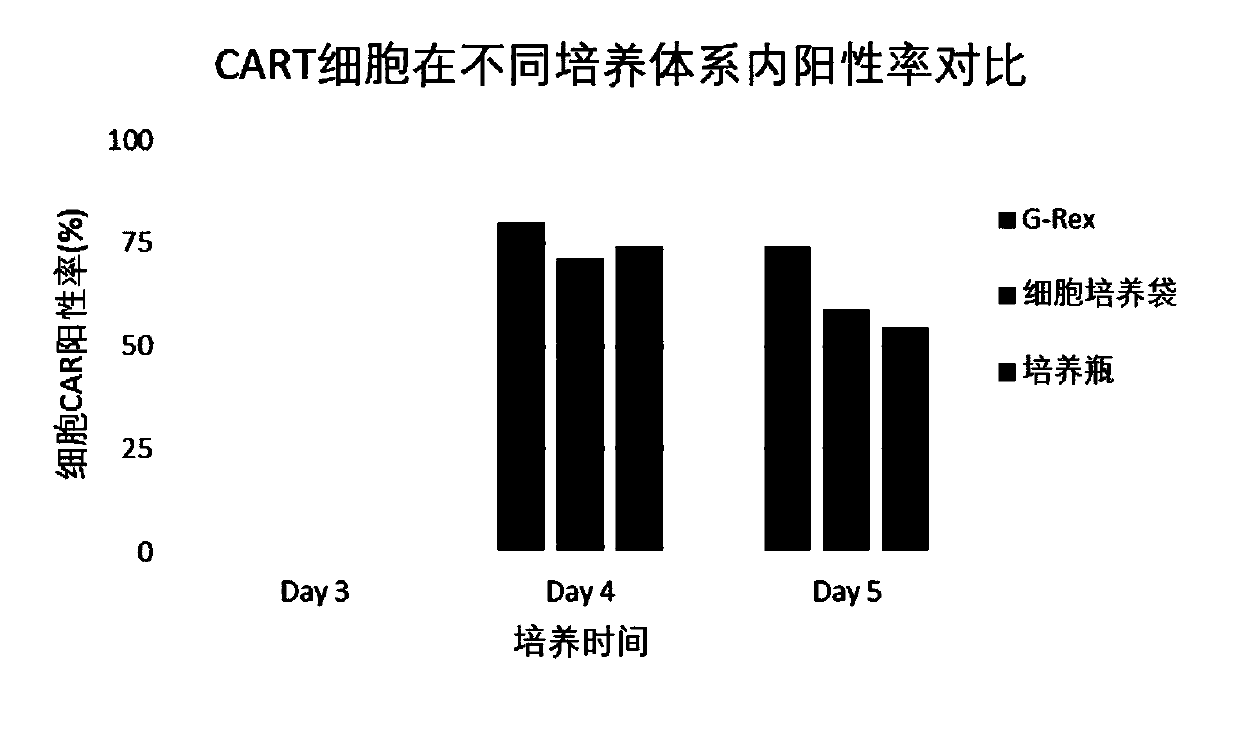
[0087] Experimental Consumables Screening
[0088] 2.1 PBMC recovery, sorting, cell activation, gene transduction
[0089] The same method as in Example 1 was used to perform cryopreserved PBMC recovery, sorting, cell activation, and gene transduction.
[0090] 2.2 Virus removal
[0091] Centrifuge the above cell suspension at 200-300 g for 6-8 minutes. Aspirate the supernatant, flick and resuspend the cells to obtain virus-depleted T cells
[0092] 2.3 Expansion culture
[0093] Transfer the virus-depleted T cells to the medium (OpTmizer SFM+1.0% albumin), add IL-2 (final concentration is 25IU / ml), and then transfer to G-Rex culture flask, culture bag and T75 culture respectively bottle, then placed at 37°C, 5% CO 2 Continue to cultivate.
[0094] After continuing to culture for 3 days, samples were taken and counted from the G-Rex culture flask, culture bag and T75 culture flask, and the cell density and cell viability were recorded. Reserve sample testing.
[0095] ...
Embodiment 3
[0101] Cell culture supplement experiment
[0102] 3.1 Method
[0103] In this embodiment, different additives IL-2, IL-7, IL-15 or their combinations were added to the cell culture medium, and 5 experimental groups were set up.
[0104] Table 1
[0105] Experimental group 1 Experimental group 2 Experimental group 3 Experimental group 4 Experimental group 5 IL-2 + - + + + IL-7 - + + + - IL-15 - + + - +
[0106] The same method as in Example 1 was used to perform cryopreserved PBMC recovery, sorting, magnetic bead labeling activation, gene transduction, magnetic bead removal, virus removal and expansion culture.
[0107] 1) The medium used is OpTmizer SFM+albumin (final concentration is 1.0%)
[0108] 2) All steps of adding IL-2 were replaced with cytokines corresponding to experimental groups 2-5 in Table 1, and cultured.
[0109] 3.2 Results
[0110] Such as Figure 4 Shown: The combination of cytokines IL-2, IL-7, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com