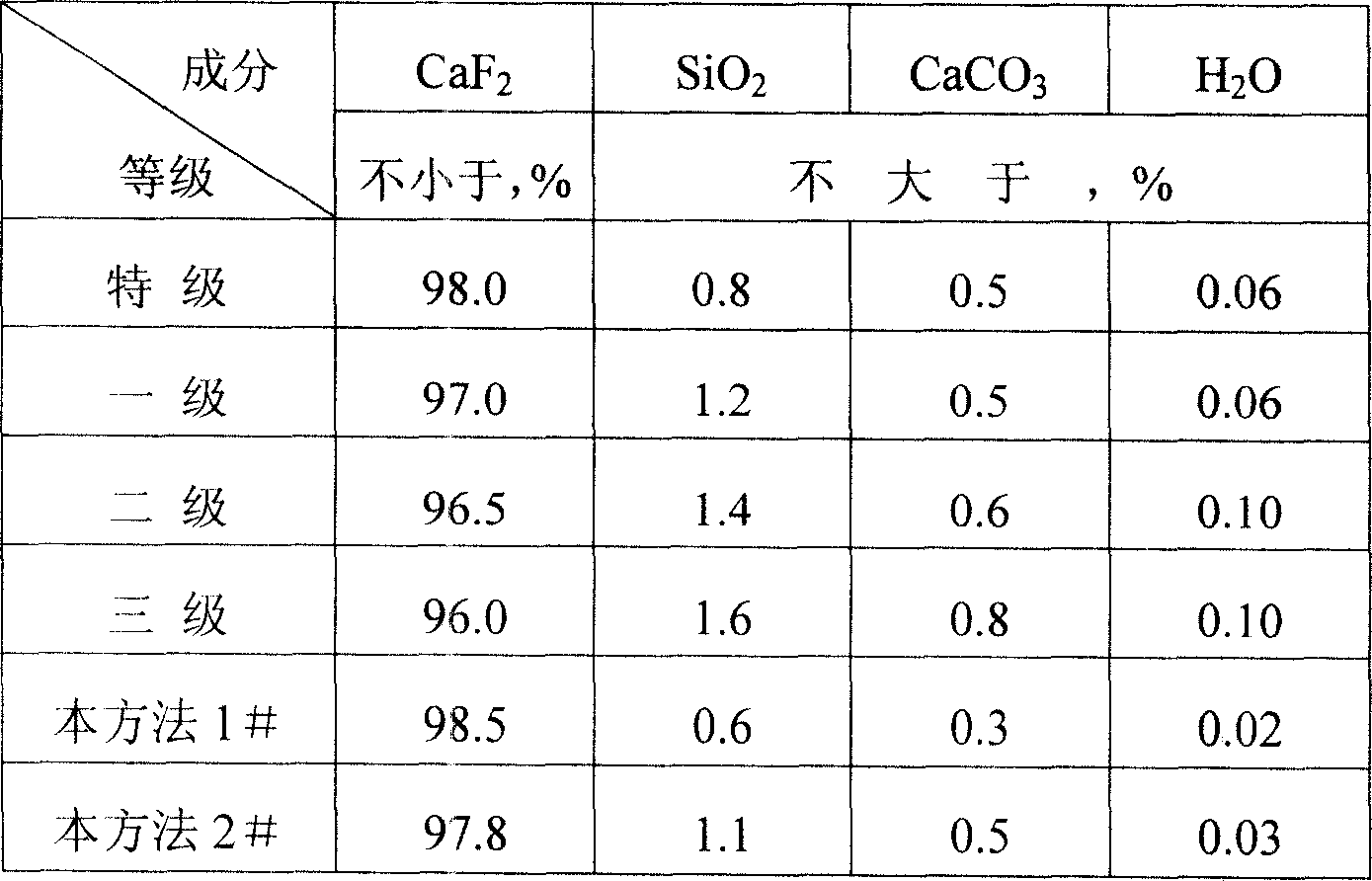
Method for producing calcium fluoride
A production method and technology of calcium fluoride, applied in the direction of calcium/strontium/barium fluoride, calcium/strontium/barium halide, etc., can solve the problems of complex production process and high production cost, and achieve good economic value and social value , cost saving, pollution mitigation effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The production method of the calcium fluoride of the present embodiment, take fluosilicic acid and calcium oxide as raw material, comprise the following steps:
[0019] (1) reacting the fluosilicic acid solution with a concentration of 15% and the calcium oxide solid for 40 minutes, after the reaction, filter to obtain the calcium fluosilicate solid;
[0020] (2) Calcium fluorosilicate is decomposed at a temperature of 300° C. for 4 hours to generate calcium fluoride solid product and silicon tetrafluoride gas;
[0021] (3) Absorb silicon tetrafluoride gas with water and hydrolyze it, filter the obtained fluorosilicic acid solution and return it to make calcium fluorosilicate, wash and dry the silicon dioxide solid to obtain white carbon black.
Embodiment 2
[0023] The production method of the calcium fluoride of the present embodiment, take fluosilicic acid and calcium oxide as raw material, comprise the following steps:
[0024] (1) reacting a 25% fluosilicic acid solution with a 30% calcium oxide slurry for 30 minutes, after the reaction, filter to obtain a calcium fluosilicate solid;
[0025] (2) Calcium fluorosilicate is decomposed at a temperature of 400°C for 3 hours to generate calcium fluoride solid product and silicon tetrafluoride gas;
[0026] (3) Absorb and hydrolyze the silicon tetrafluoride gas with water, and return the obtained fluorosilicic acid solution with a concentration of 25% after filtration to produce calcium fluorosilicate, wash and dry the silicon dioxide solid to obtain white carbon black.
Embodiment 3
[0028] The production method of the calcium fluoride of the present embodiment, take fluosilicic acid and calcium oxide as raw material, comprise the following steps:
[0029] (1) reacting a 35% fluosilicic acid solution with a 20% calcium oxide slurry for 20 minutes, after the reaction, filter to obtain a calcium fluosilicate solid;
[0030] (2) Calcium fluorosilicate is decomposed at a temperature of 500° C. for 2 hours to generate calcium fluoride solid product and silicon tetrafluoride gas;
[0031] (3) Absorb and hydrolyze silicon tetrafluoride gas with water, and return the 35% fluosilicic acid solution obtained by filtration to prepare calcium fluosilicate, wash and dry the silicon dioxide solid to obtain white carbon black.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com