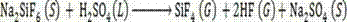Method for preparing silicon tetrafluoride and anhydrous hydrogen fluoride by taking sodium fluorosilicate as raw material
A technology of anhydrous hydrogen fluoride and silicon tetrafluoride, which is applied in the directions of fluorine/hydrogen fluoride, halogenated silicon compounds, halogenated silanes, etc. Accurate temperature control is very difficult, hydrogen fluoride gas escapes, etc., to achieve significant energy saving, avoid equipment corrosion, and fully react
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
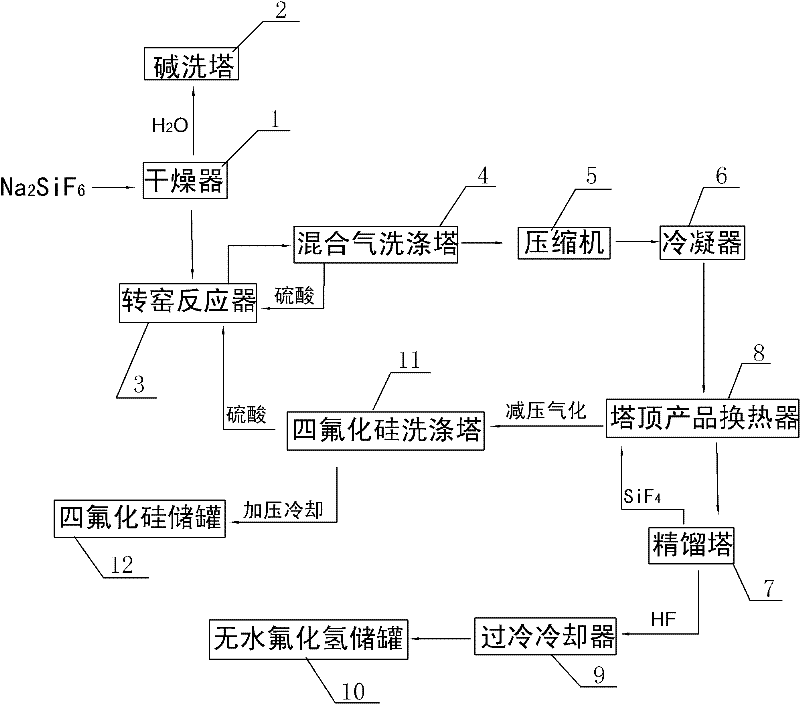
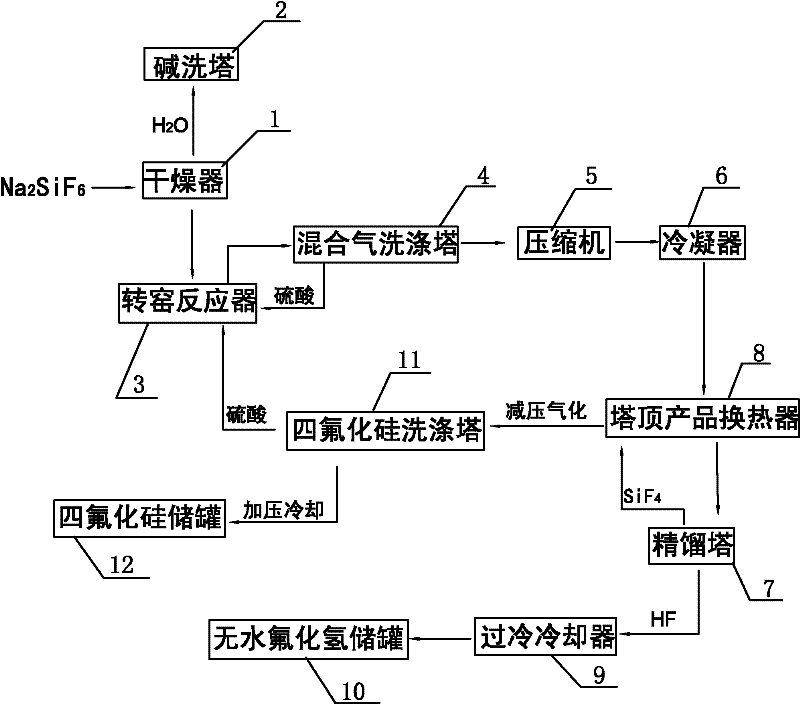
[0026] A kind of method that the present invention prepares silicon tetrafluoride and anhydrous hydrogen fluoride with sodium fluorosilicate as raw material, such as figure 1 Shown include the following steps:
[0027] (1) Dry the sodium fluorosilicate solid through the drier 1 to remove water until the mass percentage of water in the sodium fluorosilicate solid is lower than 1wt%, and send the dried water vapor into the alkali washing tower 2 with 30wt% hydrogen The sodium oxide solution is cooled and absorbed;
[0028] (2) Add sulfuric acid in 100-104wt% sulfuric acid to dried sodium fluorosilicate solid in a molar ratio of 1:1 into the rotary kiln reactor 3, and control the sodium fluorosilicate and sulfuric acid in the rotary kiln reactor 3 The mixed material is reacted at a temperature of 160-220°C for 30-180 minutes to generate sodium sulfate solid and a mixed gas of silicon tetrafluoride and hydrogen fluoride;
[0029] (3) The mixed gas of silicon tetrafluoride and hy...
Embodiment 2
[0034] Same as Example 1, the difference is that in step (3), the mixed gas of silicon tetrafluoride and hydrogen fluoride is discharged from the top of the rotary kiln reactor 3 and sent to the mixed gas scrubber 4 to remove solid dust and water vapor, and then sent to the compressor 5 pressurized to 1.0MPaG, and then pre-cooled to -25°C through the condenser 6, wherein the washing liquid of the mixed gas scrubber 4 is 98wt% sulfuric acid; in step (4), the pre-cooled silicon tetrafluoride and hydrogen fluoride The mixed gas is first cooled to -30°C through the tower top product heat exchanger 8 of the rectification tower 7, and then sent to the rectification tower 7 for separation. The operating pressure of the rectification tower 7 is controlled to be 0.7MPaG, and the pressure The temperature of the top condenser (not shown in the figure) is -75°C, and anhydrous hydrogen fluoride with a purity of 99.95wt% is extracted from the bottom of the rectification tower 7, which is coo...
Embodiment 3
[0036] Same as Example 1, the difference is that in step (3), the mixed gas of silicon tetrafluoride and hydrogen fluoride is discharged from the top of the rotary kiln reactor 3 and sent to the mixed gas scrubber 4 to remove solid dust and water vapor, and then sent to the compressor 5 pressurize to 1.5MPaG, and then pre-cool to -30°C through condenser 6, wherein the washing liquid of mixed gas scrubber 4 is 98wt% sulfuric acid;
[0037]In step (4), the pre-cooled silicon tetrafluoride and hydrogen fluoride mixed gas is first cooled to -35°C through the top product heat exchanger 8 of the rectification tower 7, and then sent to the rectification tower 7 for separation. The operating pressure of rectification tower 7 is 0.75MPaG, the temperature of the top condenser (not shown in the figure) of rectification tower 7 is controlled at -80°C, and anhydrous hydrogen fluoride with a purity of 99.95wt% is produced from the bottom of rectification tower 7 , sent to anhydrous hydrogen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com