Method for preparing silicon tetrafluoride co-production with calcium fluoride by using fluosilicic acid and calcium carbide dust
A technology of silicon tetrafluoride and calcium fluorosilicate, applied in the direction of silicon halide compound, calcium/strontium/barium fluoride, halogenated silane, etc., can solve the problems of difficult removal of impurities, many by-products, low conversion rate, etc. Achieve the effect of low cost of raw materials, saving raw materials and solving environmental problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
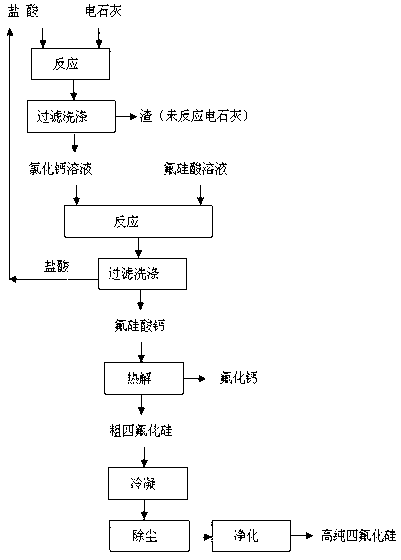
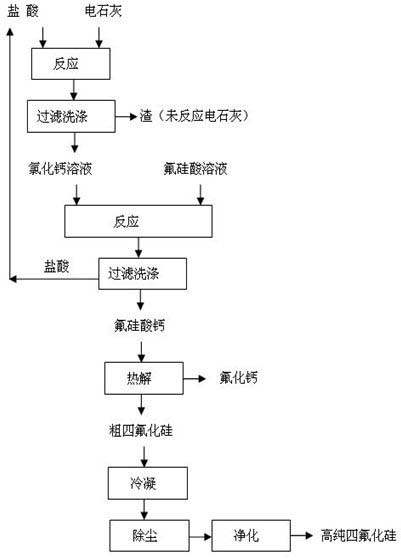
[0023] The method for preparing silicon tetrafluoride and co-producing calcium fluoride by using fluosilicic acid and calcium carbide, the specific steps are:
[0024] (1) 100g of calcium carbide and 815g of hydrochloric acid with a mass fraction of 10% were reacted at room temperature (25°C) for 6h, filtered and washed, and 35g of the wet filter residue was unreacted calcium chloride solution; After concentrating, the obtained mass fraction is 300g of calcium chloride solution of 40%;
[0025] (2) 300 g of calcium chloride solution with a mass fraction of 40% and 830 g of a fluorosilicic acid solution with a mass fraction of 15% were reacted for 5 hours at room temperature according to the molar ratio of calcium chloride to fluorosilicate acid 1.25:1.0, filtered and washed, filtered After the cake is dried, 150g of calcium fluosilicate is obtained, and the filtrate hydrochloric acid is recycled;
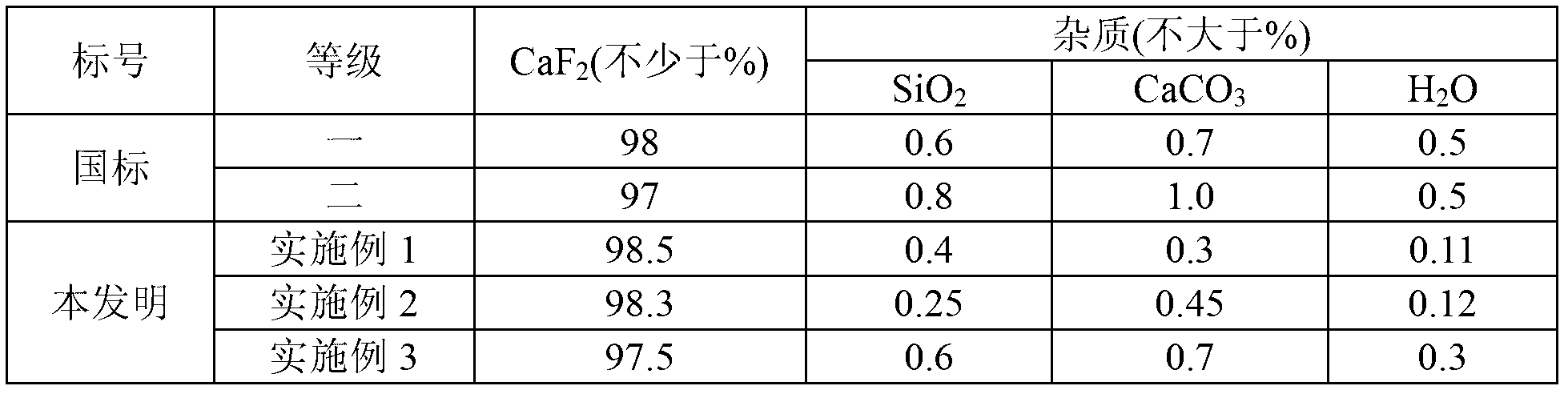
[0026] (3) pyrolyze 150g of calcium fluorosilicate at 300°C for 6 hours to obt...
Embodiment 2
[0030] The method for preparing silicon tetrafluoride and co-producing calcium fluoride by using fluosilicic acid and calcium carbide, the specific steps are:
[0031] (1) React 100 g of calcium carbide with 550 g of filtrate hydrochloric acid with a mass fraction of 15% at 50 ° C for 4 hours, filter and wash, and 32 g of wet filter residue is unreacted calcium chloride solution; the filtrate and washing liquid are calcium chloride solution, after concentration The obtained mass fraction is 280g of calcium chloride solution of 45%;
[0032] (2) 280g of calcium chloride solution with a mass fraction of 45% and 420g of a fluorosilicic acid solution with a mass fraction of 30% were reacted for 3 hours at room temperature according to the molar ratio of calcium chloride to fluorosilicate acid 1.3:1.0, filtered and washed, filtered After the cake is dried, 155g of calcium fluosilicate is obtained, and the filtrate hydrochloric acid is recycled;
[0033] (3) 155g of calcium fluoros...
Embodiment 3
[0037] The method for preparing silicon tetrafluoride and co-producing calcium fluoride by using fluosilicic acid and calcium carbide, the specific steps are:
[0038] (1) React 100 g of calcium carbide with 550 g of filtrate hydrochloric acid with a mass fraction of 15% at 100 ° C for 2 hours, filter and wash, and 30 g of wet filter residue is unreacted calcium chloride solution; the filtrate and washing liquid are calcium chloride solution, after concentration The obtained mass fraction is 250g of calcium chloride solution of 50%;
[0039] (2) 250 g of calcium chloride solution with a mass fraction of 50% and 315 g of a fluorosilicic acid solution with a mass fraction of 40% were reacted for 1 hour at room temperature according to the molar ratio of calcium chloride to fluorosilicate acid of 1.5:1.0, filtered and washed, filtered After the cake is dried, 157g of calcium fluosilicate is obtained, and the filtrate hydrochloric acid is recycled;
[0040] (3) 157g of calcium fl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com