Method for producing monoammonium phosphate and magnesium ammonium phosphate by using wet concentrated phosphoric acid residues
A technology of monoammonium phosphate and phosphoric acid residue acid, applied in the chemical industry, can solve the problems of conventional product backlog, low economic value, affecting product quality, etc., and achieve high added value and good economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
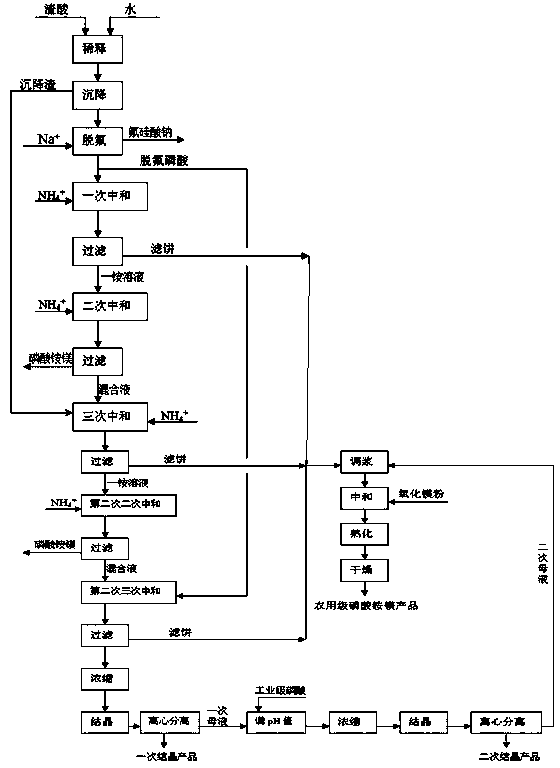
[0040] A. Take 3000 grams of wet concentrated phosphoric acid residue from a phosphate fertilizer factory (including: P 2 O 5 43.04%; F1.99%; SO 3 6.65%; CaO 3.15%; MgO 2.12%; Fe 2 O 3 3.30%; AL 2 O 3 2.21%; 2.20% solids), add 3000 grams of water, stir and dilute, stir well and let it settle for 24 hours. 4165 grams of clear dilute acid was obtained by siphoning, and the sedimentation residue at the bottom was left for use.
[0041]B. In the clarified dilute acid obtained in step A, slowly add 21 grams of technical-grade sodium carbonate, fully react under normal temperature stirring for 1 hour, filter and separate the filtrate and filter residue, wash the filter residue with water to no acidity, and dry to obtain 21 grams Sodium Fluorosilicate Products (Na 2 SiF 6 content 95.10%), the washing water was incorporated into the defluorinated phosphoric acid filtrate to obtain 4185 grams of defluorinated dilute phosphoric acid;
[0042] C, get 3315 grams of defluorina...
Embodiment 2
[0051] A. Take 3000 grams of wet concentrated phosphoric acid residue from a phosphate fertilizer factory (including: P 2 O 5 43.04%; F1.99%; SO 3 6.65%; CaO 3.15%; MgO 2.12%; Fe 2 O 3 3.30%; AL 2 O 3 2.21%; 2.20% solids), add 3000 grams of water, stir and dilute, stir well and let it settle for 24 hours. 4165 grams of clear dilute acid was obtained by siphoning, and the sedimentation residue at the bottom was reserved for use.
[0052] B, in the clarified dilute acid obtained in step A, slowly add 16 grams of technical grade sodium hydroxide, fully react under normal temperature stirring for 1 hour, filter and separate the filtrate and the filter residue, wash the filter residue with water to no acidity, and dry to obtain 21 grams of sodium fluosilicate products (Na 2 SiF 6 content 95.02%), the washing water was incorporated into the defluorinated phosphoric acid filtrate to obtain 4192 g of defluorinated dilute phosphoric acid;
[0053] C, get 3370 grams of defl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com