Method for producing fluorine series compounds and white carbon black
A production method, a technology for silica, applied in chemical instruments and methods, silicon compounds, alkali metal fluorides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
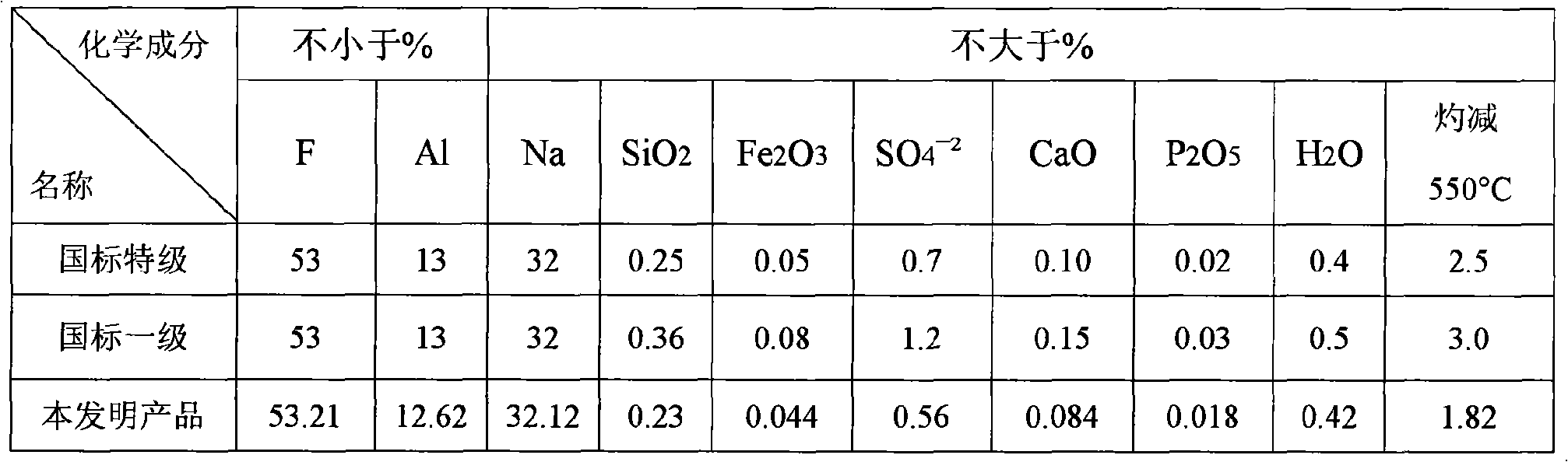
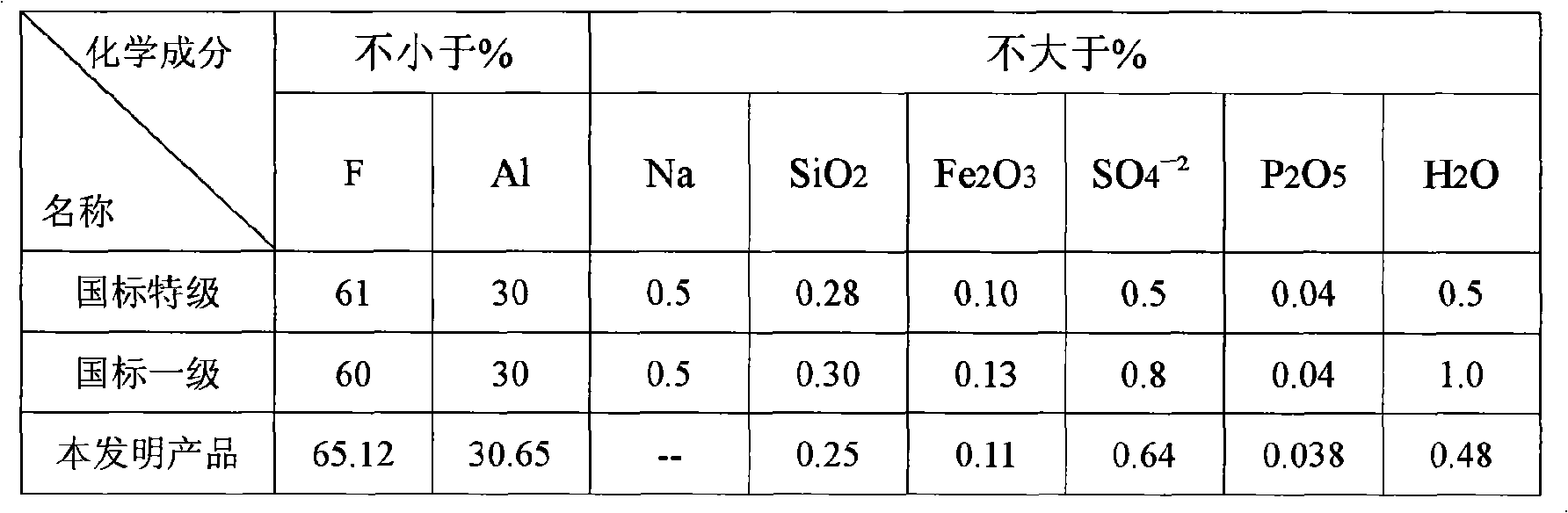
[0088] Bring the concentration to ~20% NH 4 The F solution is introduced into the exhaust gas absorption system of the chemical formation chamber with an annual output of 100,000 tons of ordinary calcium for absorption, and the concentration ~ 34% (NH 4 ) 2 SiF 6Solution, add it and liquid ammonia to the ammoniator at the same time and control its pH value at 7.2-7.5, then pass liquid ammonia into the ammoniator and control the final pH value of the ammoniation reaction to be above 8, and the temperature at 50-70 ℃, the obtained slurry is aged for 1 hour and then filtered, washed countercurrently with clean water until the eluate is close to neutral, and the filter cake is dried at 110 ℃ to obtain a specific surface area of 110m 2 / g of precipitated silica (silica) product and a concentration of 40.6% NH 4 F in ammonium fluoride solution. Such as ~ 34% (NH 4 ) 2 SiF 6 When the solution is introduced into a container with a stirring blade and cooled to ~ 20°C, ammonium...
Embodiment 2
[0090] Ready 700g 32% NH 4 F solution and 200 g 99% Na 2 SiF 6 . In a reactor equipped with a stirrer, first add ~150 g of 32% NH 4 F solution, then slowly add ~ 43 grams of sodium fluorosilicate at a temperature of 65 ° C, and then add the remaining ammonium fluoride solution and sodium fluorosilicate in proportion and uniformly, the entire feeding time is 1 hour, and then add After finishing, continue to stir and react for half an hour. Control a certain rate to put the reaction slurry into a pre-filled with a small amount of 32% NH 4 In the ammoniator with stirring of the F solution, feed ammonia to keep the pH value of the liquid phase in the ammoniator at pH 7.2 to 7.5. After the reaction slurry is added, continue to pass ammonia to increase the pH value of the liquid phase. High and keep the pH above 8, while controlling the slurry temperature in the range of 50-70°C. Stop stirring after the ammoniation is finished, settle naturally, release the upper suspension, t...
Embodiment 3
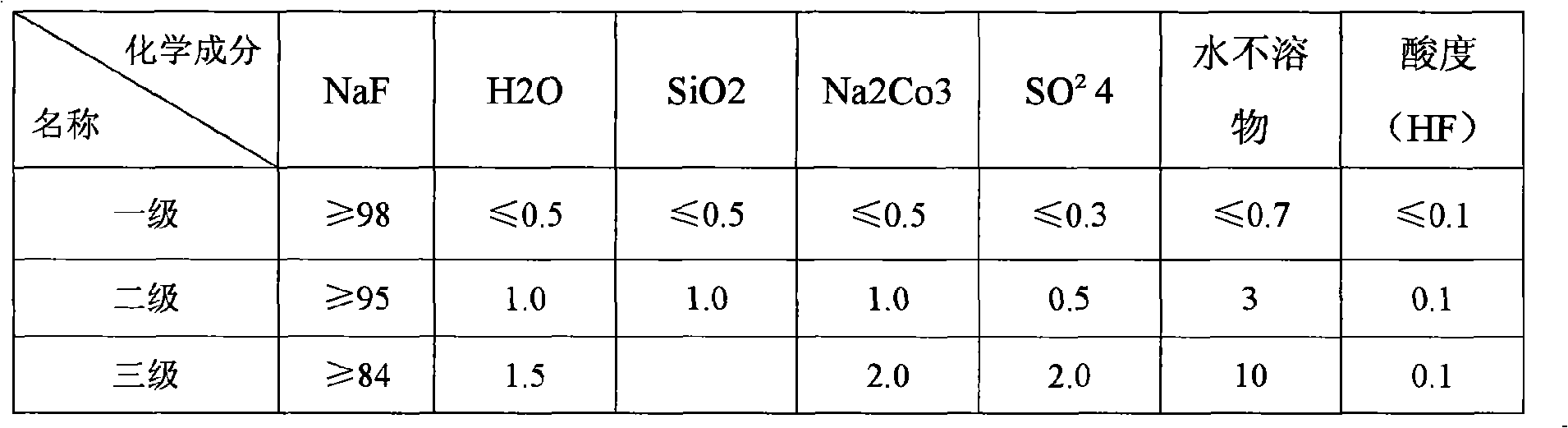
[0092] Set the flow rate to 1.46m 3 / hr concentration 410g / lNH 4 ammonium fluoride solution and a flow rate of 3.12m 3 Sodium chloride solution with a concentration of 25.5% NaCl / hr was added to the first reactor, and after ~45 minutes of reaction, it overflowed into the second reactor, and at the same time, 0.94m 3 / hr25.5% NaCl sodium chloride solution, the reaction material overflows into the thickener after staying in the second reactor for 30 minutes, and the thick phase is filtered, washed, and dried to obtain sodium fluoride products, and the dilute phase and filtrate Contains 53.9g / l sodium chloride and 164.4g / l ammonium chloride. It can be separated by thermal separation method. Ammonium chloride is used as a by-product, and sodium chloride is returned to participate in the reaction. It is entrusted to use ARL9800XP+ X-fluorescence spectrometer for analysis. The results are shown in the table:
[0093] Table 1. Sodium Fluoride product quality of the present inventi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com