Technique for preparing waterless hydrogen fluoride on high purity
An anhydrous hydrogen fluoride, high-purity technology, applied in the direction of fluorine/hydrogen fluoride, etc., can solve the problems of low industrial value of calcium sulfate, difficult to control the molar ratio, and harsh filtration environment, so as to achieve low product cost, reduce repair costs, and process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
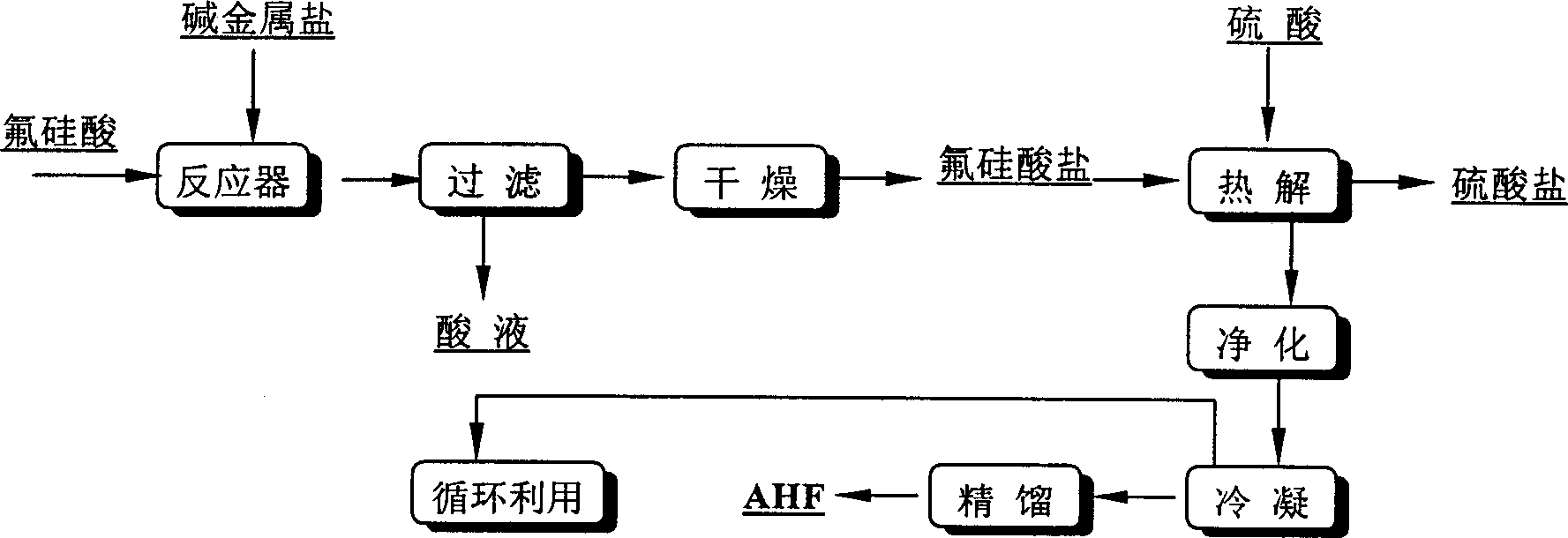
[0045] Take 5000 kg of 15% fluosilicic acid, add 816 kg of potassium chloride with a purity of 95%, the reaction temperature is 40° C., the time is 1 hour, and the liquid-solid ratio is controlled at 4.3. Filter after completion of the reaction, the filter cake weighs 1077 kg, and the filtrate contains 8.1% hydrogen chloride.
[0046] After mixing 1077 kg of precipitated potassium fluorosilicate with 490 kg of 98% sulfuric acid, they were calcined at 500° C. for 40 minutes. 874 kg of potassium sulfate was produced with a purity of 97.6%; the generated gas was condensed at -5°C for 3 hours and separated to obtain 176 kg of anhydrous hydrogen fluoride with a purity of 99.93%. Silicon tetrafluoride is cyclically dissolved in water to produce fluosilicic acid. The weight of fluosilicic acid is 5040 kg, and the concentration is 10%.
Embodiment 2
[0048] Take 2000 kg of 15% fluosilicic acid, add 380 kg of potassium sulfate with a purity of 94.6%, the reaction temperature is 40° C., the time is 1 hour, and the liquid-solid ratio is controlled at 4.1. Filter after completion of the reaction, the filter cake weighs 439.6 kg, and the filtrate contains 10.5% sulfuric acid.
[0049] After mixing 439.6 kg of potassium fluorosilicate with 195.5 kg of 98% sulfuric acid, it was calcined at 450° C. for 1 hour. 358.7 kg of potassium sulfate was produced with a purity of 95.2%; the generated gas was condensed at -5°C for 2 hours to obtain 73.8 kg of anhydrous hydrogen fluoride with a purity of 99.96%. Silicon tetrafluoride is cyclically dissolved in water to produce fluosilicic acid. The weight of fluosilicic acid is 1506 kg, and the concentration is 13.3%.
Embodiment 3
[0051] Take 5000 kg of 15% fluosilicic acid, add 750 kg of sodium sulfate with a purity of 98.2%, the reaction temperature is 40° C., the time is 1 hour, and the liquid-solid ratio is controlled at 6.0. Filter after completion of the reaction, the filter cake weighs 764.2 kg, and the filtrate contains 10.2% sulfuric acid.
[0052] After mixing 764.2 kg of sodium fluorosilicate with 402 kg of 98% sulfuric acid, it was calcined at 450° C. for 1 hour. 581 kg of sodium sulfate was generated with a purity of 97.34%. The generated gas was condensed at 0°C for 3 hours and separated to obtain 151.4 kg of anhydrous hydrogen fluoride with a purity of 99.98%. Silicon tetrafluoride is cyclically dissolved in water to produce fluosilicic acid. The weight of fluosilicic acid is 3680 kg and the concentration is 11.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com