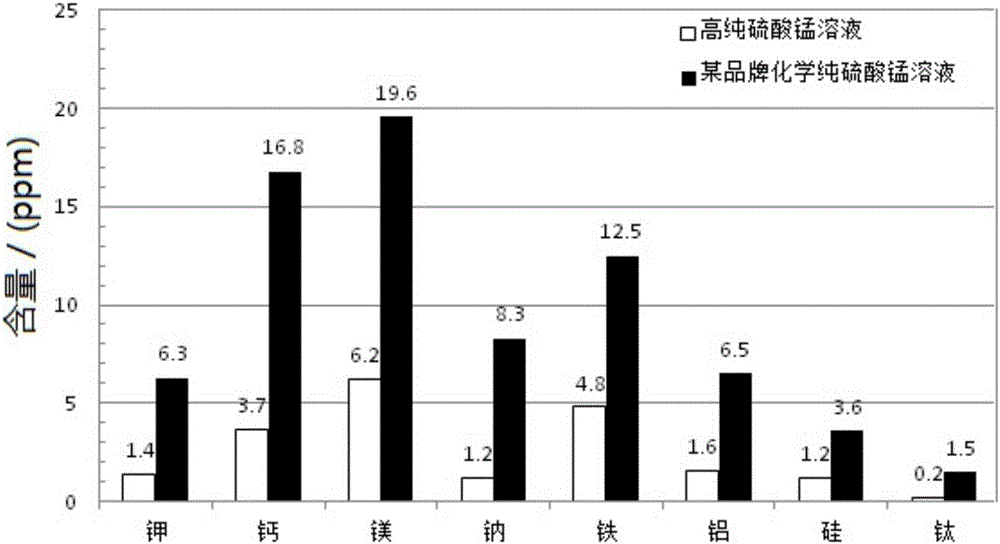
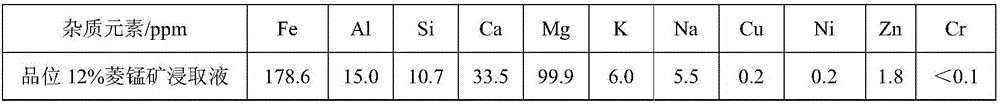
Patents
Literature
104 results about "Barium sulfide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Barium sulfide is the inorganic compound with the formula BaS. BaS is an important precursor to other barium compounds including BaCO₃ and the pigment lithopone, ZnS/BaSO₄. Like other chalcogenides of the alkaline earth metals, BaS is a short wavelength emitter for electronic displays. It is colorless, although like many sulfides, it is commonly obtained in impure colored forms.
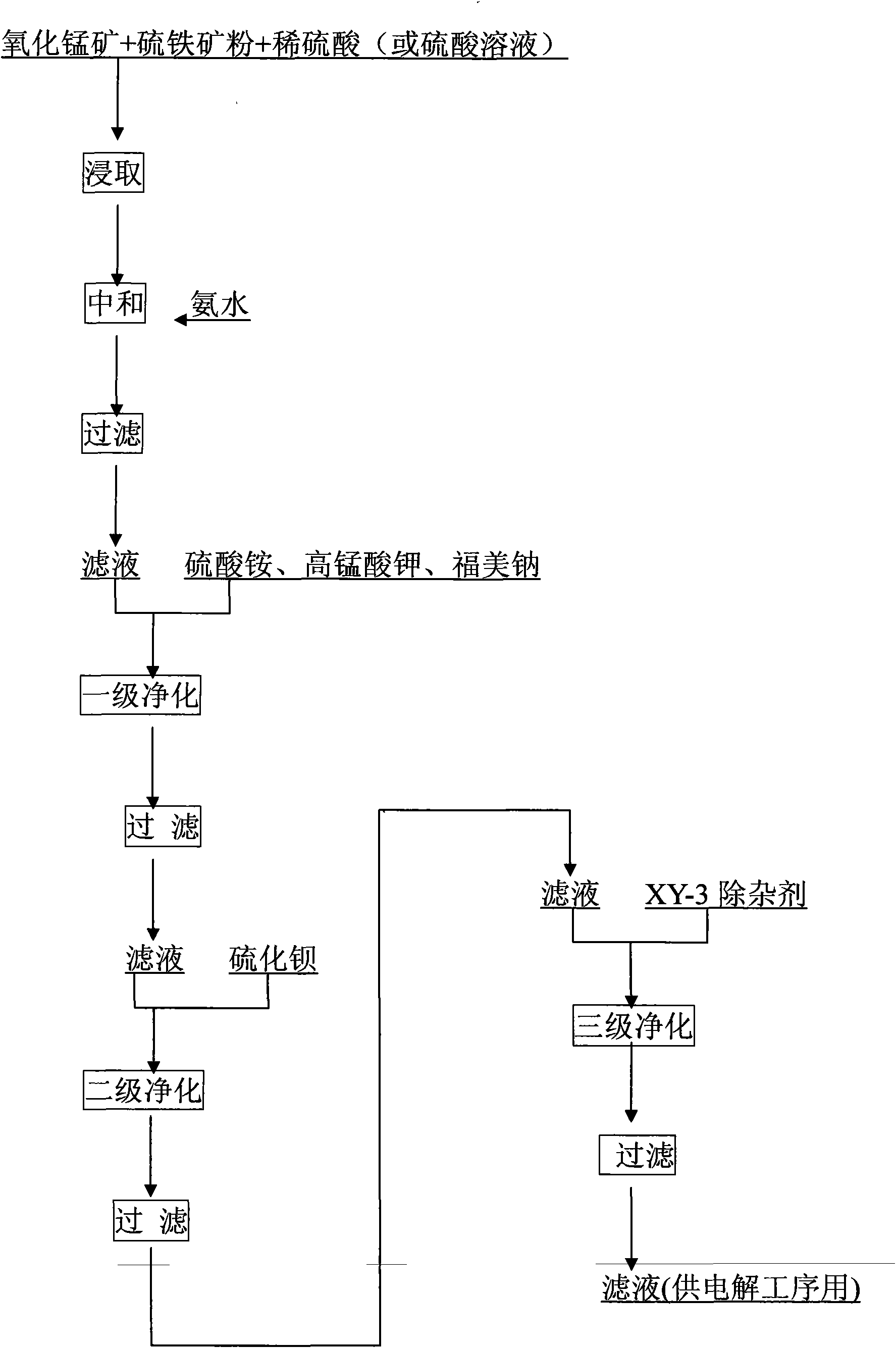
Liquid making technique for manganese oxide ore
InactiveCN101684562AAchieve outputRealization costPhotography auxillary processesElectrolysisFiltration
The invention discloses a liquid making technique for manganese oxide ore, comprising the steps of: adding the manganese oxide ore powder, pyrite powder, sulfuric acid solution into a leaching vat forleaching; adding a neutralizing agent for neutralizing after reaching leaching termination; adding ammonia sulfate, potassium permanganate and sodium dimethyldithiocarbamate into the neutralization liquid after filtration until the nickel is qualitatively removed; then, adding barium sulfide or adding barium sulfide into the filtrate after filtration, wherein the dosage of barium sulfide is 100-2000g / m<3> manganese sulfate solution; adding XY-3 impurity removal agent after filtration, wherein the dosage of XY-3 impurity removal agent is 50-400g / m<3> manganese sulfate solution, the temperatureis controlled at 30-80 DEG C, the reaction time is 0.5-3h, and the pH is 4.5-6.9; and filtrating to obtained purified manganese sulfate solution. The recovery rate of the qualified, refined and purified manganese sulfate solution is more than 90%, based on common electrolytic technique, the quality of products achieves national standard, the electrolytic yield is larger than 3kg / veneer, the powerconsumption is not more than 7500kwh / t, and the yield, cost and efficiency are all better than the traditional level of manganous carbonate production.
Owner:熊一言
Increasing thermal conductivity of host polymer used with laser engraving methods and compositions
InactiveUS7980596B2Improve security levelReduce chancePhotosensitive materialsPretreated surfacesIodideSodium iodide
the invention provides a composition having laser engraving properties, comprising a host material and a laser enhancing additive. The host material comprises a material, such as a polymer, modified by a first process, whereby the host material as modified by the first process has increased thermal conductivity as compared to the host material before the first process. The laser enhancing additive comprises a first quantity of at least one of copper potassium iodide (CuKI3), Copper Iodide (CuI), potassium iodide (KI), sodium iodide (NaI), and aluminum iodide (AlI), and a second quantity of at least one substance selected from the group consisting of zinc sulfide (ZnS), barium sulfide (BaS), alkyl sulfonate, and thioester.
Owner:L 1 SECURE CREDENTIALING
Method for preparing barium sulfate and zinc sulfide
InactiveCN101205077AIncrease profitImprove protectionCalcium/strontium/barium sulfatesZinc sulfidesSulfateMirabilite
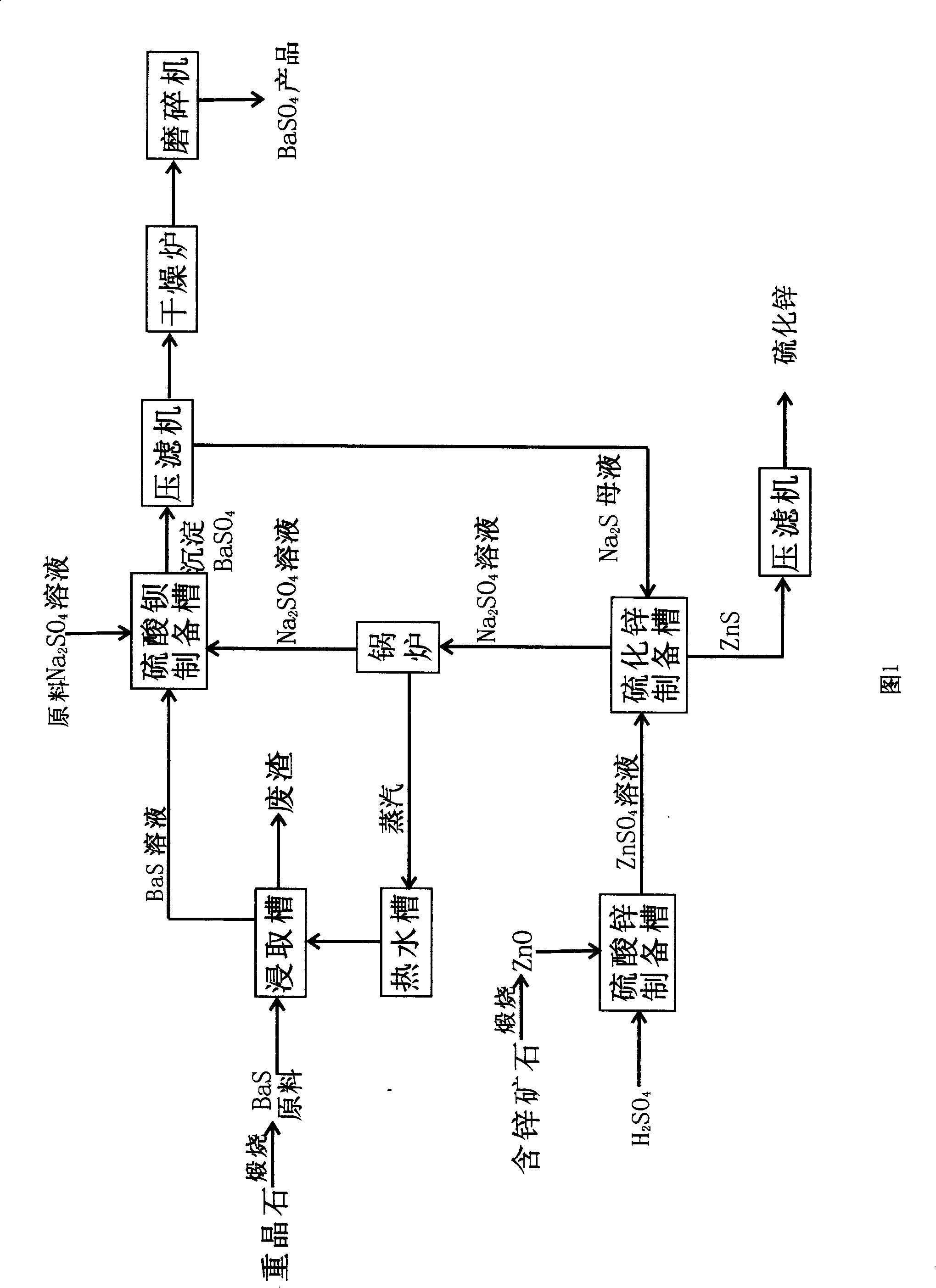
The invention discloses a method for preparing barium sulfate and zinc sulphide, which takes barites and zinc containing ore as principal material and includes the following steps: firstly, black ash raw material which contains more than 50% of barium sulphide prepared by the mixed calcinations of barites and coal is leached to get pellucid barium sulphide solution; secondly, the barium sulphide solution reacts with mirabilite to get barium sulfate products after filter pressing separation, drying and grinding; thirdly, zinc oxide obtained by the calcinations of the zinc containing ore reacts with sulphuric acid, and zinc sulfate solution is prepared by the purification of reacting solution; fourthly, depurative zinc sulfate solution reacts with vulcanized alkali containing mother liquor which is prepared after the separation of the barium sulfate from the reacting resultant obtained from step two to get zinc sulphide after filter pressing separation; the sodium sulfate containing mother liquor which is separated from zinc sulphide is put into a boiler for concentration; the steam formed heats the leach barium sulfide water; the concentrated sodium sulfate containing solution reacts with barium sulfide to prepare precipitated barium sulfate.Due to the adoption of closed cycle, the invention has the advantages of simple operation, high yield, energy conservation and environmental protection.
Owner:LUOYANG HONGYUAN BARIUM SALT CHEM IND INST
Increasing thermal conductivity of host polymer used with laser engraving methods and compositions
InactiveUS7728048B2Improve thermal conductivityImproved grayscale engravingPhotosensitive materialsPattern printingSodium iodideIodide
the invention provides a composition having laser engraving properties, comprising a host material and a laser enhancing additive. The host material comprises a material, such as a polymer, modified by a first process, whereby the host material as modified by the first process has increased thermal conductivity as compared to the host material before the first process. The laser enhancing additive comprises a first quantity of at least one of copper potassium iodide (CuKI3), Copper Iodide (CuI), potassium iodide (KI), sodium iodide (NaI), and aluminum iodide (AlI), and a second quantity of at least one substance selected from the group consisting of zinc sulfide (ZnS), barium sulfide (BaS), alkyl sulfonate, and thioester.
Owner:L 1 SECURE CREDENTIALING
Increasing Thermal Conductivity of Host Polymer Used With Laser Engraving Methods and Compositions
InactiveUS20100181754A1Improve security levelReduce chancePhotosensitive materialsPretreated surfacesThioester synthesisSodium iodide
the invention provides a composition having laser engraving properties, comprising a host material and a laser enhancing additive. The host material comprises a material, such as a polymer, modified by a first process, whereby the host material as modified by the first process has increased thermal conductivity as compared to the host material before the first process. The laser enhancing additive comprises a first quantity of at least one of copper potassium iodide (CuKI3), Copper Iodide (CuI), potassium iodide (KI), sodium iodide (NaI), and aluminum iodide (AlI), and a second quantity of at least one substance selected from the group consisting of zinc sulfide (ZnS), barium sulfide (BaS), alkyl sulfonate, and thioester.
Owner:L 1 SECURE CREDENTIALING
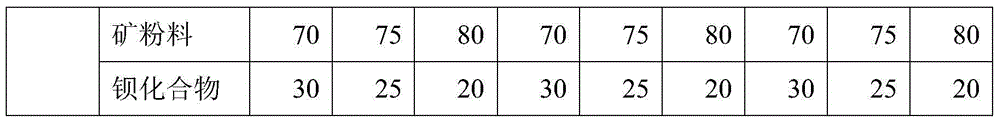
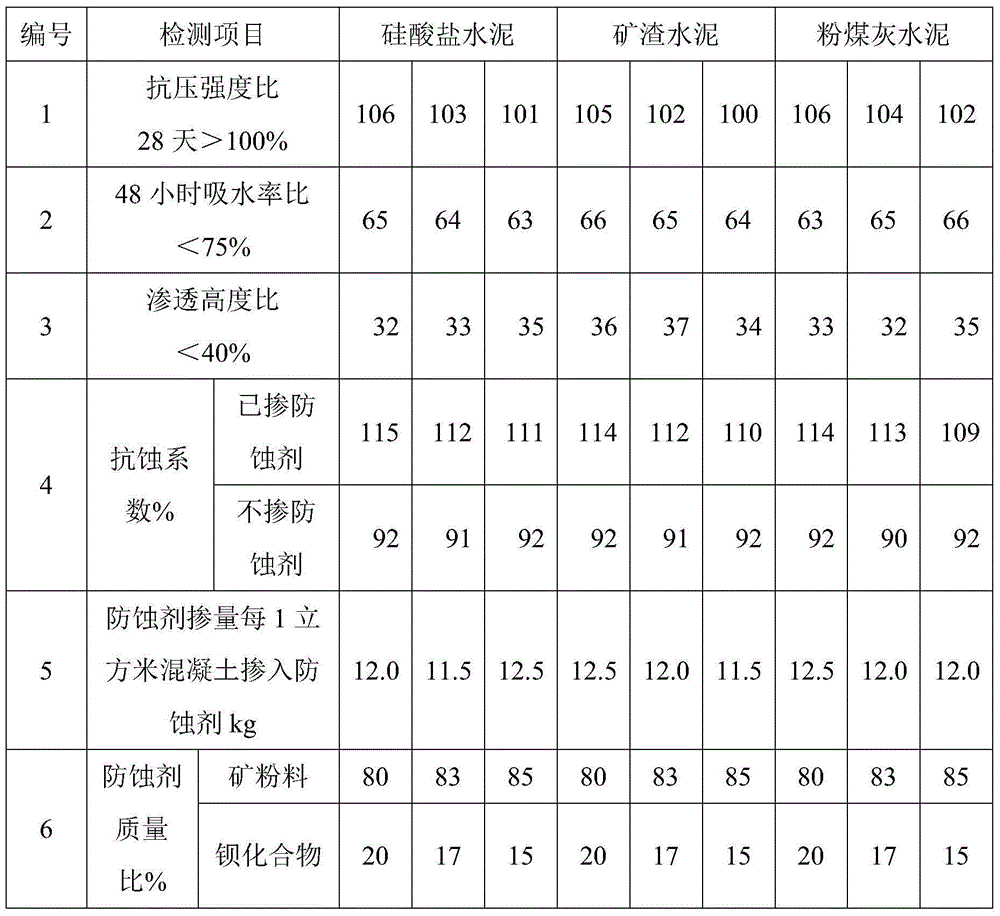
Anticorrosive agent for concrete and anticorrosive concrete
The invention provides an anticorrosive agent for concrete. The anticorrosive agent is a mixture of powder selected from the group consisting of fly ash, blast-furnace water-quenched slag powder, silica fume, light-burnt metakaolin powder, phosphorus slag powder, alum slag powder, natural zeolite powder and the like and a barium compound selected from the group consisting of barium chloride, barium nitrate, barium acetate, barium hydroxide, barium sulfide and the like. The anticorrosive agent for concrete provided by the invention enables Ca(OH)2 by-produced in hydration of cement to bond with SiO2 included in cement so as to produce secondary hydrated calcium silicate, so the strength and impermeability of concrete are improved; barium ions doped into concrete reacts with SO4<2-> from the outside in time to produce inertial and stable secondary barite, so deionization of SO4<2-> is realized and corrosivity of SO4<2-> is eradicated; the secondary barite forms a stable mineral inorganic protection film on the surface layer of concrete, so further invasion of external harmful ions is prevented, the strength, impermeability and corrosion resistance of concrete are further improved, the service life of concrete is substantially prolonged, and economic benefits are increased.
Owner:李亚铃
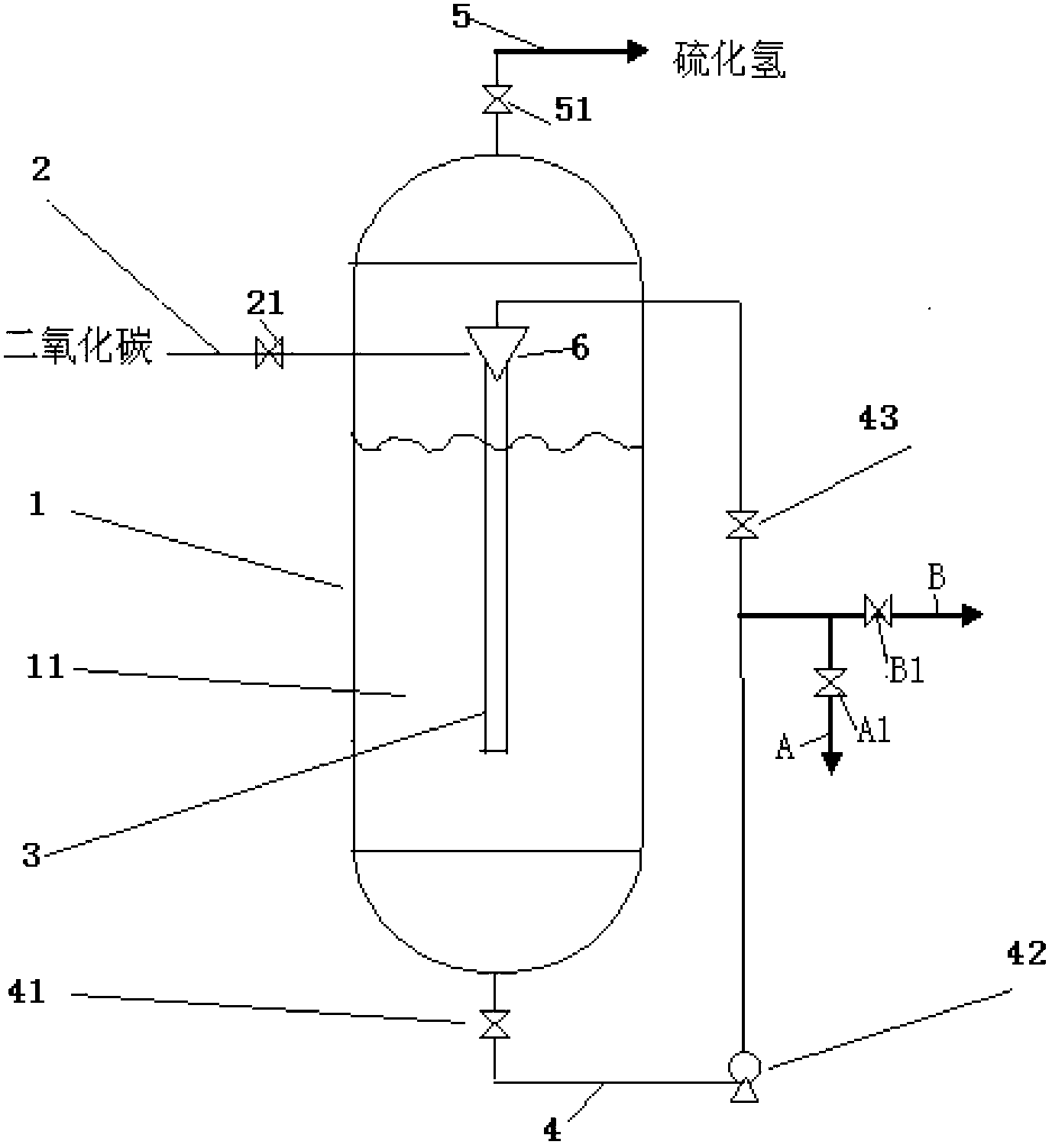
Treatment process and device of waste gas containing hydrogen sulfide and carbon dioxide
InactiveCN102059038AHigh puritySolve the problem of waste gas utilizationCalcium/strontium/barium carbonatesDispersed particle separationEnvironmental resistanceBarium sulfide
The invention discloses treatment process and device of a waste gas containing hydrogen sulfide and carbon dioxide. The treatment process comprises the following steps of: firstly decarbonizing the waste gas through a multilevel barium sulfide solution; then carrying out alkali wash through a sodium hydroxide solution, and then emptying; separating sediments generated after decarbonization to obtain a barium carbonate product; recycling mixed liquor generated after the decarbonization; and evaporating, crystallizing and separating a solution obtained by alkali wash to obtain a sodium sulfide product. The treatment process and device provided by the invention have simple process flow, cannot only directly produce the barium carbonate by utilizing CO2 contained in a mixed gas, but also produce an H2S gas needed by next step reaction through reaction, thereby not only consuming the carbon dioxide gas contained in the mixed gas, but also obtaining a hydrogen sulfide gas with very high purity; and in addition, the crystal sodium sulfide is produced by using the hydrogen sulfide, therefore, no matter the environmental protection and the benefits are optimized.
Owner:LIAOCHENG LUXI CHEM ENG DESIGN
Method for treating vanadium extracting industrial acid wastewater and comprehensively recycling valuable metal
ActiveCN105567976AControl parameters are clearly definedEasy to controlCalcium/strontium/barium sulfatesCement productionWastewaterBarium sulfide
The invention discloses a method for treating vanadium extracting industrial acid wastewater and comprehensively recycling valuable metal. The method comprises the following steps that aeration and oxidation are carried out on the acid wastewater, and then a purifying agent A is added and is at least one of limestone, unslaked lime, slaked lime and lime milk; stirring is carried out for reacting, and the pH of the reaction endpoint is controlled to be equal to 4.5 to 5.0; filtering and washing are carried out, and primary purifying liquid and primary purifying residues are obtained; a purifying agent B is added to the primary purifying liquid, and is at least one of unslaked lime, slaked lime and lime milk; stirring is carried out for reacting, and the pH of the reaction endpoint is controlled to be equal to 6.5 to 7.5; filtering and washing are carried out, and secondary purifying liquid and secondary purifying residues are obtained; and a purifying agent C is added to the secondary purifying liquid and is at least one of sodium dimethyldithiocarbamate (SDD), sodium sulfide and barium sulfide, stirring is carried out for reacting, filtering is carried out, and third purifying liquid and third purifying residues are obtained. The method has the beneficial effects of being simple in process step, low in treatment cost, good in valuable metal comprehensive recovery effect and the like.
Owner:SHENGTONG YIHE TIANJIN ENTERPRISE MANAGEMENT CONSULTING CO LTD
Barium sulfate composite particles, resin composition containing same, and process for producing same
ActiveCN104411757AFunction increaseIncreased durabilityMaterial nanotechnologyPigmenting treatmentZinc compoundsBarium sulfide
[Problem] With respect to barium sulfate which is for use in electronic appliances as a filler for resin compositions, precipitated barium sulfate synthesized from barium sulfide as a starting material has a problem that since the barium sulfate contains sulfur as an impurity, use thereof in a resin composition for an ink, film, sheet, or the like that is for use in an electronic appliance results in a possibility that the hydrogen sulfide which has volatilized from the barium sulfate might deteriorate or corrode metallic portions, e.g., electrodes, of electronic components, thereby impairing the function, durability, and reliability of the electronic appliance. [Solution] Barium sulfate composite particles which comprise barium sulfate particles and a zinc compound adhered to the surface thereof and which have an average particle diameter of 0.01-10 µm.
Owner:SAKAI CHEM IND CO LTD
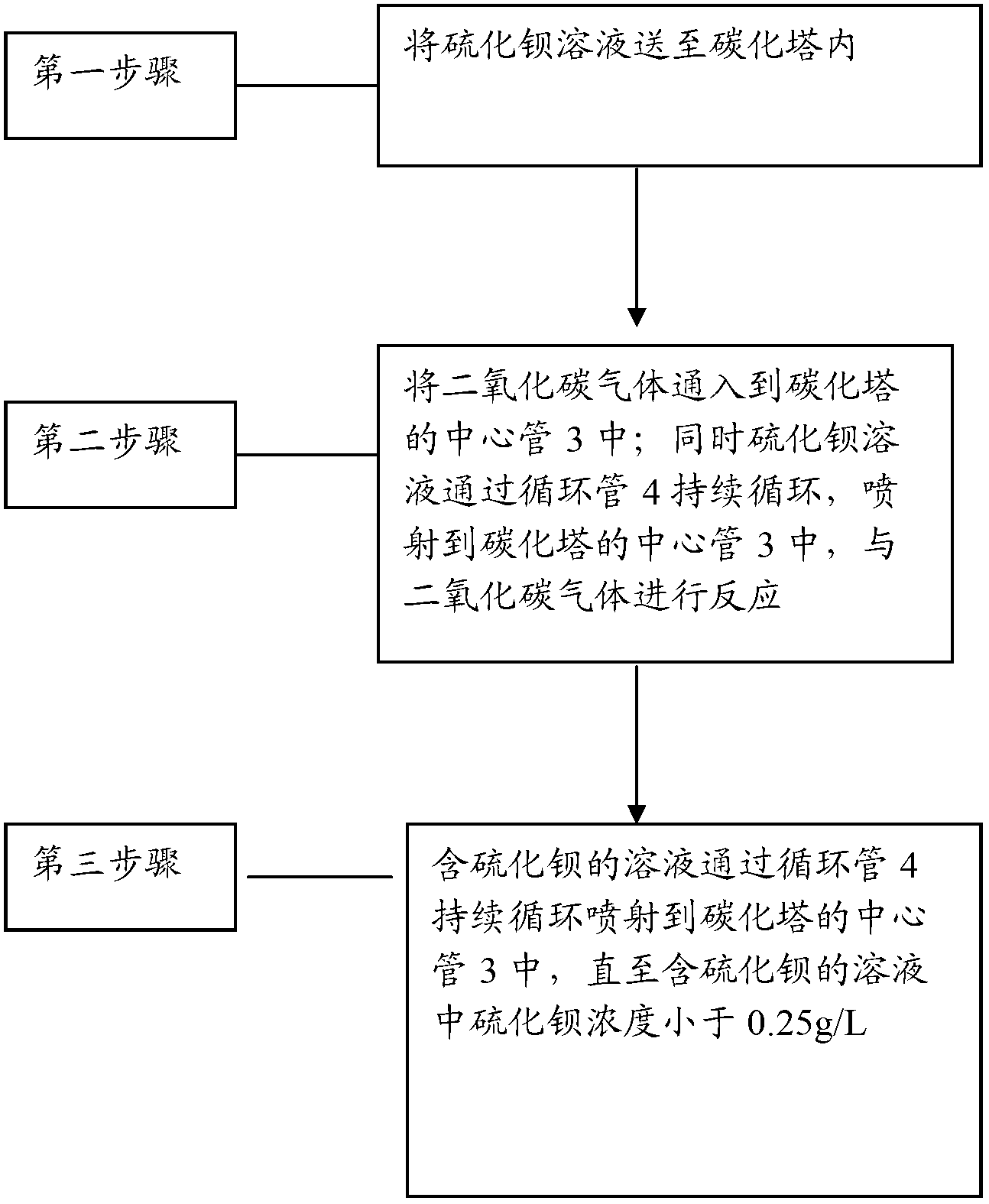
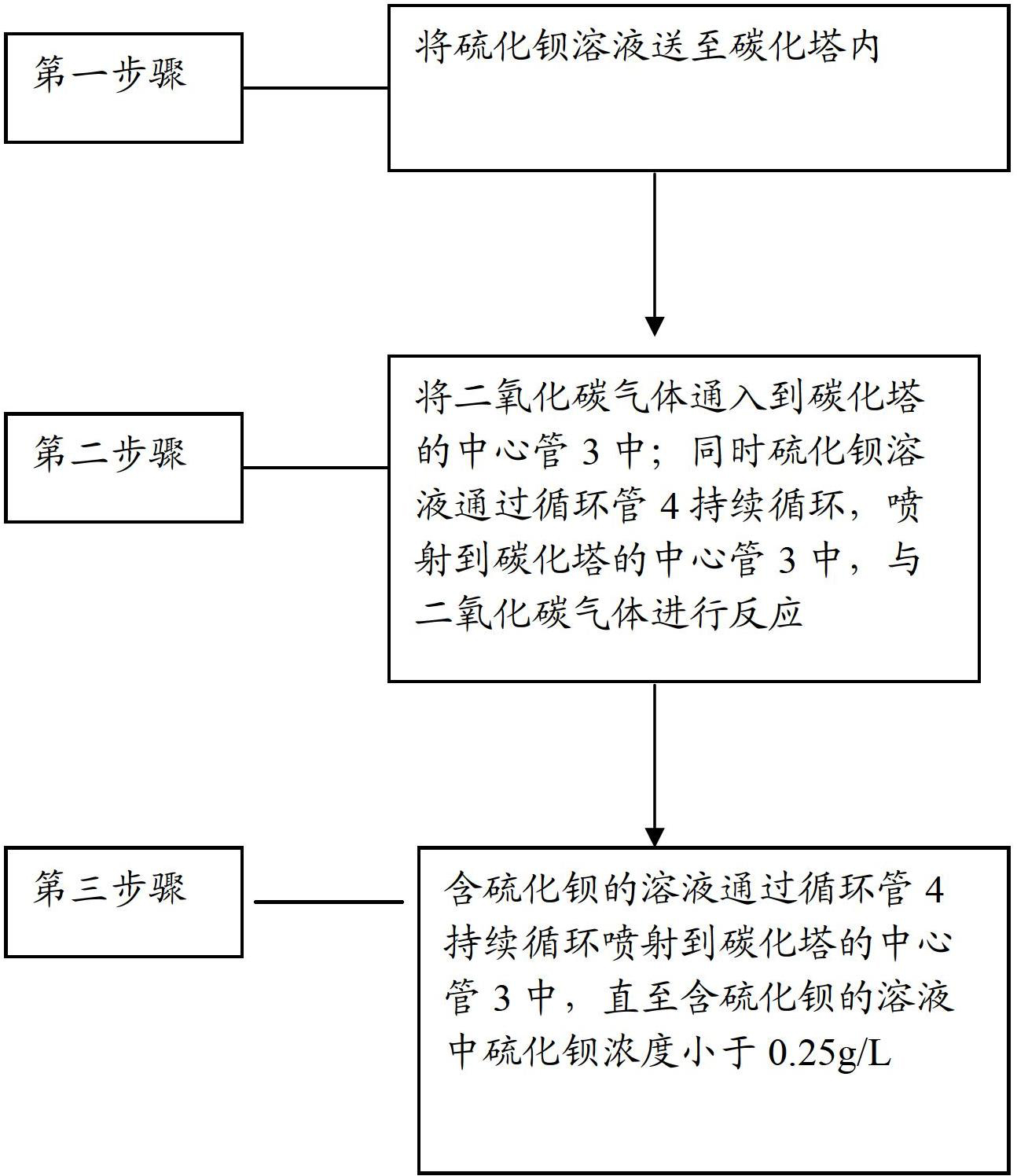
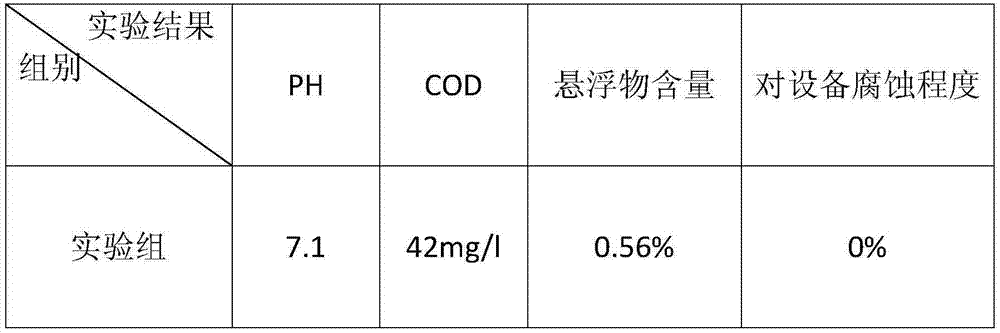
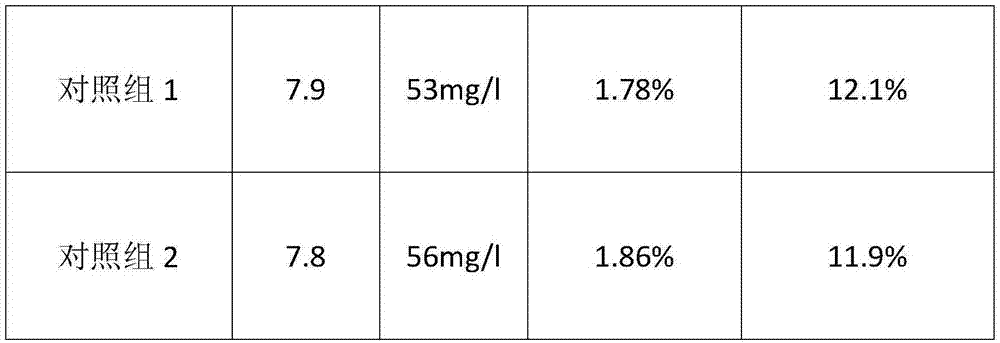
Barium carbonate, preparation method thereof and carbonizer
ActiveCN102701252ARapid responseThe rate of reaction nucleation increasesCalcium/strontium/barium carbonatesSolubilityBarium sulfide
The invention relates to barium carbonate, a preparation method of the barium carbonate and a carbonizer. The preparation method of barium carbonate comprises the steps of: (1) conveying barium sulfide solution to the carbonizer, wherein the barium sulfide solution has the solubility of 120-170g / L at the temperature of 65-75 DEG C; (2) introducing carbon dioxide gas to a central tube (3) of the carbonizer, and simultaneously spraying the barium sulfide solution to the central tube (3) of the carbonizer to react with carbon dioxide gas; and (3) persistently and circularly spraying the solution containing barium sulfide to the central tube (3) of the carbonizer by a circulating pipe (4), stopping reaction until the barium sulfide concentration in the solution containing barium sulfide is smaller than 0.25g / L, and carrying out solid-liquid separation to obtain the barium carbonate product. According to the barium carbonate, a preparation method of the barium carbonate and a carbonizer provided by the invention, the barium sulfide solution is sprayed by single tower body internal circulation of the carbonizer, so that rapid reaction is carried out between the barium sulfide solution and the carbon dioxide gas, the reaction nucleation rate is increased and the crystal development and growth is slow, therefore, obtained barium carbonate has narrow particle size distribution, and the reaction activity is increased to more than 85%.
Owner:GUIZHOU REDSTAR DEVING
Method for co-production of feed manganese sulfate by using wastewater of crude indium production
ActiveCN103613141AReduce dosageReduce manufacturing costManganese sulfatesEcological environmentIndium
The invention discloses a method for co-production of feed manganese sulfate by using wastewater of crude indium production. According to the method, on the basis of production of feed manganese sulfate through a wet method, the influence of excessive sulfuric acid and heavy metals including arsenic, cadmium and the like in the wastewater of the crude indium production through a wet method on the environment is thoroughly eliminated while the feed manganese sulfate is produced, so that the production of crude indium and the feed manganese sulfate through the wet method achieves comprehensive and full utilization of wastes, and the pollution caused by waste acid water containing heavy metals to the environment is thoroughly eliminated. The method comprises the steps of 1, performing leaching reaction on manganese ash in a leaching tank by using indium preparation wastewater; 2, performing pressure filtration; 3, adjusting the pH value of the filtrate to 4-4.5, and then pressing liquid; 4, adding barium sulfide to improve the pH value of a stock solution to 5-5.4, and then filtering; 5, cooling and standing the stock solution, adding an SDD (sodium dimethyl dithiocarbamate) solution and a flocculating agent, then performing pressure filtration, concentrating the filtrate, performing evaporative crystallization, and drying to obtain a feed manganese sulfate product. By adopting the method, the indium preparation wastewater and waste residues causing environmental pollution can be fully and comprehensively used, and a raw material for producing manganese sulfate is provided, so that the method is favorable for reducing the production cost and protecting the ecological environment.
Owner:JIANGXI RUIDA NEW ENERGY TECH CO LTD
Preparation method of heavy metal sewage treatment medicament
InactiveCN104445550AUniversalEasy to handleWaste water treatment from quariesWater contaminantsReaction temperatureTreatment costs
The invention provides a preparation method of a heavy metal sewage treatment medicament. The preparation method comprises the following steps: preparing raw materials including barium sulfide, ferric sulfate, polymeric silicate aluminum sulfate, calcium hydroxide and lithopone; adding barium sulfide, ferric sulfate and polymeric silicate aluminum sulfate into a reactor; stirring and heating to 120 DEG C, and carrying out backflow for 6-9 hours while maintaining the reaction temperature at 110-130 DEG C; adding water to dilute calcium hydroxide, and adequately stirring the solution with dried jelly; carrying out boiling reaction at 100 DEG C, so as to prepare a mother solution; filtering the mother solution after the reaction to obtain filtrate for later use, and recycling filter residues; cooling the mother solution at the room temperature, adding lithopone into the reactor, uniformly stirring until initial condensation; heating at 60-80 DEG C for 1-3 hours, stirring for 15 minutes, and using at 5-50 DEG C. The heavy metal sewage treatment medicament is wide in application range, good in sedimentation effect, water purification quality, dehydration property, compression property and effluent quality and low in treatment cost and can serve as a dehydration flocculant.
Owner:南京新美汇电子科技有限公司
Sewage treatment agent and preparing method thereof
ActiveCN105439220AFast purificationImprove purification effectWater treatment parameter controlWater/sewage treatmentPotassium persulfateSodium Bentonite
The invention discloses a sewage treatment agent and a preparing method thereof. The sewage treatment agent is prepared from, by weight, 30-36 parts of kaolin, 28-32 parts of bentonite, 26-28 parts of seaweed meal, 22-24 parts of activated carbon, 20-24 parts of aluminum ash, 20-22 parts of poly-silicon aluminum sulfate, 18-24 parts of citric acid, 18-22 parts of polyferric chloride, 14-18 parts of zinc acetate, 18-20 parts of sodium carbonate, 16-18 parts of potassium persulfate, 14-18 parts of sodium hydroxide, 20-22 parts of chitosan, 22-24 parts of barium sulfide and 18-22 parts of dimethyl diallyl. The sewage treatment agent is environmentally friendly, free of toxicity, high in purification speed and good in purification effect.
Owner:潍坊农丰宝环境科技有限公司
Submicron flake-shaped barium sulfate and preparation method thereof
ActiveCN103693671ASolve technical problems of preparationHigh purityCalcium/strontium/barium sulfatesFiltrationSulfur
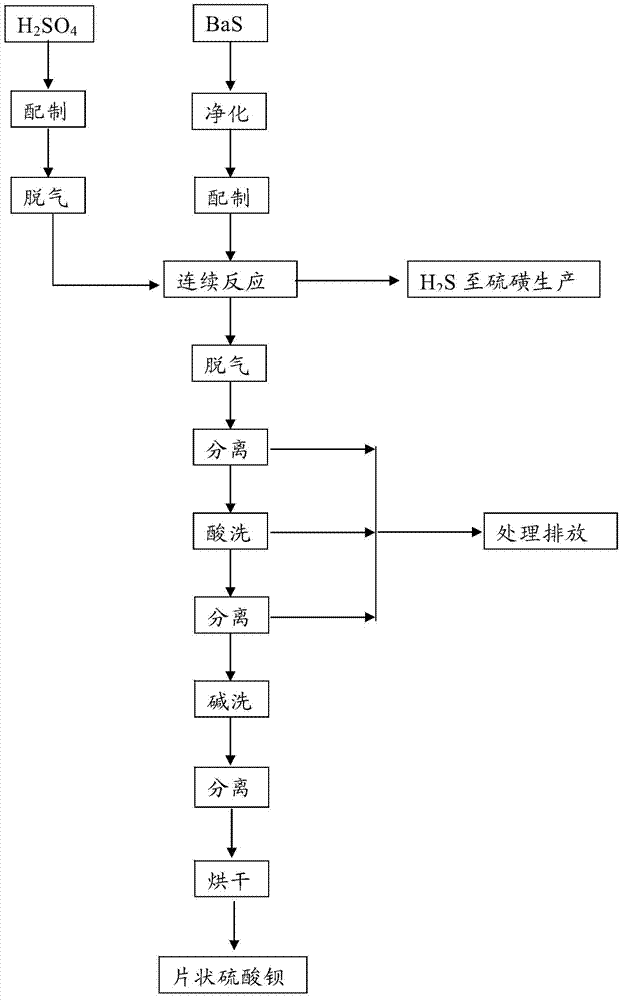
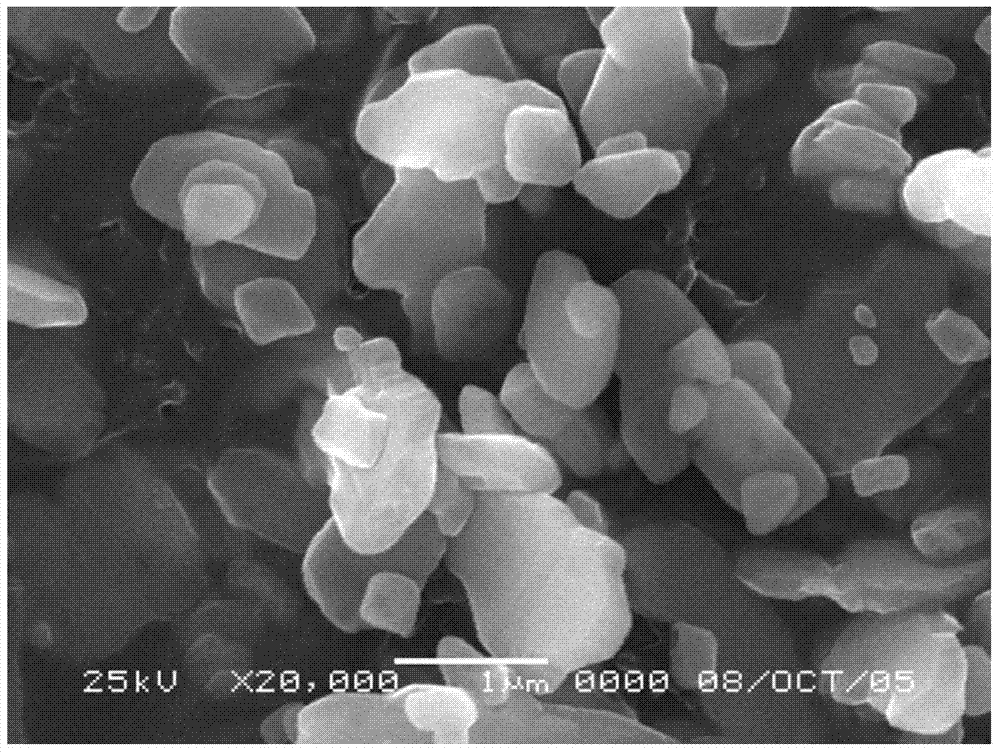
The invention discloses a submicron flake-shaped barium sulfate and a preparation method thereof. The preparation method comprises the following steps: (1) pretreating barium sulfate, namely, preparing sulfur into a sulfur aqueous solution with the concentration of 4.0+ / -0.5mol / L, degassing under stirring, and subsequently cooling and filtering so as to obtain pretreated sulfuric acid; (2) pretreating barium sulfide, namely, performing hot filtration on the barium sulfide solution with the concentration of 60-90g / L so as to obtain pretreated barium sulfide; (3) continuously synthesizing, namely, stirring to react the obtained BaS solution with the sulfuric acid obtained in the step (1) in the flow of 600+ / -50L / h, and keeping the concentration of the sulfuric acid in the reaction solution be greater than 3.0g / L in the reaction process so as to obtain reaction slurry; (4) performing an aftertreatment procedure; and (5) performing a finished product procedure so as to obtain the barium sulfate product. The barium sulfate is flake-shaped, the barium sulfate content is within 99.4-99.5%, the whiteness is greater than 99.5%, and D50 is within the range of 0.70-0.80 micron.
Owner:HONGXING XINHUANG EXACT CHEM
Anti-corrosion brake pad and preparing method of brake pad
The invention relates to the technical field of brake pads. The brake pad comprises raw materials including, by weight, 20 parts to 25 parts of ceramic fibers, 5 parts to 10 parts of carbon fibers, 5 parts to 10 parts of steel fibers, 15 parts to 18 parts of modified phenolic resin, 6 parts to 10 parts of calcium oxide, 6 parts to 8 parts of barium sulfide, 15 parts to 20 parts of crystalline flake graphite, 1 part to 3 parts of zirconite, 5 parts to 10 parts of butyl butylbenzene, 10 parts to 15 parts of butyronitrile powder, 3 parts to 5 parts of molybdenum disulfide, 1 part to 2 parts of a bonding agent, 5 parts to 10 parts of blanc fixe, 0.5 part to 3 parts of zinc oxide, 5 parts to 10 parts of rubber powder, 1 part to 2 parts of fluorite powder and 0.5 part to 1 part of silicon carbide. The invention further provides a preparing method of the anti-corrosion brake pad. The method comprises the steps that resin and other powdery materials are put to be mixed and stirred, the fiber materials are added for stirring, hot pressing is carried out, and the brake pad formed through pressing is subject to heat treatment. The brake pad has the beneficial effects of being good in heat resistance, resistant to wear, high in toughness and not prone to corrosion.
Owner:杭州西湖摩擦材料有限公司
Heavy metal wastewater treatment disinfectant
InactiveCN103803666AReduce processing costsEasy to useWater/sewage treatment by neutralisationWater/sewage treatment by flocculation/precipitationCalcium hydroxideDisinfectant
The invention discloses a heavy metal wastewater treatment disinfectant. The heavy metal wastewater treatment disinfectant is prepared from the following raw materials in percentage by weight: 30-50% of barium sulfide, 10-30% of calcium carbonate, 10-30% of calcium hydroxide and 20-30% of ferrous sulfate. According to the heavy metal wastewater treatment disinfectant provided by the invention, the problems that similar existing products are insufficient and the heavy metal wastewater treatment is difficult are solved, the sources of the raw materials are wide, and the costs of the raw materials are low; when the heavy metal wastewater treatment disinfectant is applied to the heavy metal wastewater treatment, the use is convenient, meanwhile, the cost is low, the pollution caused by the heavy metal wastewater can be effectively solved, the discharge water of enterprises is helped to meet a national discharged standard, and the environmental pollution is avoided.
Owner:JIANGXI YUANXIN RESOURCE RECYCLING INVESTMENT DEV
Production method for electrolytic manganese metal
ActiveCN105154917AReduce manufacturing costPrevent splashPhotography auxillary processesElectrolysisManganese
The invention provides a production method for electrolytic manganese metal and relates to the technical field of producing manganese metal through electrolysis. The method comprises the procedures of the preparation of an ore pulp, iron removal through neutralization, impurity removal through purification, electrolysis, passivation, stripping and cleaning. During impurity removal through purification, after impurity removal through purification is conducted once with the adoption of sodium dimethyl dithiocarbamate (SDD), impurity removal is further conducted with the adoption of a deep impurity removal agent. The deep impurity removal agent is composed of, by weight, 10%-20% of poly-ferric aluminous sulfate, 18%-20% of potassium oxalate, 10%-15% of polyacrylamide, 10%-15% of acrylamide, 8%-10% of dimethylamine, 15%-20% of barium sulfide, 7%-15% of ethylenediamine tetraacetic acid, and 3%-4% of activated carbon. The method is suitable for producing the electrolytic manganese metal with low-grade manganese ore as raw materials; manganese ore resources are used reasonably; the production cost is reduced; the adopted process has the advantage of a high metal leaching rate, and the purity of a manganese sulfate solution obtained by using the process of deep impurity removal is high; the quality of the electrolytic manganese product is ensured.
Owner:靖西市大西南锰业有限公司
Heavy metal sewage treatment particles and preparation method thereof
InactiveCN105731580AReduce pollutionClean waterWater contaminantsWater/sewage treatment using germicide/oligodynamic-processCelluloseEpoxy
The invention discloses heavy metal sewage treatment particles and a preparation method thereof. The heavy metal sewage treatment particles are prepared from the following materials in parts by weight: 11-23 parts of activated carbon, 8-16 parts of chitosan, 14-26 parts of epoxy resin, 10-18 parts of meerschaum, 7-13 parts of smectite, 9-15 parts of alginic acid, 6-14 parts of gelatin, 15-25 parts of alum, 5-10 parts of barium sulfide, 8-14 parts of calcium carbonate, 11-16 parts of cellulose, 4-8 parts of nano titanium dioxide, 6-14 parts of hydrotalcite, 13-19 parts of silica gel and 9-19 parts of sodium sulfhydrate. The heavy metal sewage treatment particles can quickly precipitate heavy metals, and can sterilize and clean the water source.
Owner:张锐
Manufacturing process for producing synthetic reducing agent by using waste slag of electrolytic manganese and application of synthetic reducing agent
InactiveCN101880773AEmission reductionEfficient reductionProcess efficiency improvementElectrolysisFiltration
The invention relates to a manufacturing process for producing a synthetic reducing agent by using waste slag of electrolytic manganese and application of the synthetic reducing agent. The manufacturing process comprises the following steps of: adding manganese slag into mixed liquid of sulfuric acid and lixivium and performing pressure filtration after a reaction is finished to obtain the waste slag and feed liquid 1; b, mixing solution of barium sulfide with ammonium sulfate in a mass ratio of barium sulfide to ammonium sulfate of 1:(0.4-0.5), and performing pressure filtration after reaction and removing precipitate to obtain feed liquid 2; and c, adding the feed liquid 1 into the feed liquid 2, performing pressure filtration to separate out the synthetic reducing agent after a reaction is finished and the lixivium, and then recycling the lixivium into the step a. The synthetic reducing agent has the advantages of efficiently reducing and utilizing manganese dioxide ore, using the waste slag of the electrolytic manganese as a main raw material, largely consuming the waste manganese, and largely reducing the waste slag and carbon dioxide generated in the manufacturing process for leaching and producing the electrolytic manganese by using manganese carbonate and reducing the manganese dioxide ore and by other reduction methods.
Owner:张安良 +1
Barium sulfate composite particle, resin composition comprising the same, and production method thereof
InactiveUS20150111992A1Function increaseIncreased durabilityMaterial nanotechnologyPigmenting treatmentZinc compoundsBarium sulfide
As barium sulfate which is used as a filler of a resin composition for an electronic equipment, a precipitated barium sulfate which is synthesized from barium sulfide contains a sulfur component as an impurity so that metal parts such as electrodes of electronic parts maybe deteriorated and corroded by a hydrogen sulfide component volatilized from the barium sulfate to reduce the function, the durability, and the reliability of electronic equipment when used for resin compositions such as an ink, a film, and a sheet which are used for an electronic equipment.Barium sulfate composite particles having a zinc compound adhered to the particle surface and having an average particle diameter of 0.01 to 10 μm.
Owner:SAKAI CHEM IND CO LTD
Titania composite and preparing method thereof
InactiveUS20110253012A1Good chemical stabilityGood weather resistancePigmenting treatmentLiquid surface applicatorsBarium sulfideRutile
A titania composite and a preparing method thereof are provided. The titania composite comprises an inner core and a coating film layer, wherein the inner core is a barium sulfate crystal and / or a strontitum sulfate crystal with subsphaeroidal shape, having a particle size of less than 1 μm and whiteness of more than 98%, and the coating film layer is nano rutile titania. The preparing method thereof comprises the following steps: (a) preparing barium sulfate or strontium sulfate; (b) coating treatment; (c) subjecting the titania composite to a high-temperature heat treatment; and (d) subjecting the titania composite to a post treatment. The subsphaeroidal titania composite is obtained by chemically synthesizing a subsphaeroidal submicron barium sulfide or strontium sulfide, and then hydrolyzing and coating thereon to form a coating film layer of titania to make titania grow evenly. The particle size of the titania composite is small, and the particle size distribution range of the titania composite is controllable.
Owner:GUIZHOU REDSTAR DEVING
Short-process low-consumption high-purity manganese sulfate solution preparing method
The invention relates to a method for directly preparing a high-purity manganese sulfate solution from low grade rhodochrosite sulfuric acid extraction liquid. The rule that the PH value of a system is changed from small to large is implemented, and an efficient chemical coupling mechanism is played. The method includes the steps that firstly, manganese mineral powder is continuously dissolved into low grade manganese ore sulfuric acid extraction liquid, the pH value of the extraction liquid is sequentially adjusted with barium hydroxide to reach 3 to 4, then barium fluoride is added, and filtering is carried out; secondly, the pH value of filter liquor is adjusted with barium hydroxide to reach 5 to 6, then barium sulfide is added, filtering is carried out, and new filter liquor is obtained; and finally, the PH value of the new filter liquor is adjusted with barium hydroxide to reach 8, and filtering is carried out to obtain the high-purity manganese sulfate solution. The method is simple in operation, medicament waste caused by operation for repeatedly adjusting the PH value of the system in an overlapped manner is avoided, the manganese content in the prepared high-purity manganese sulfate solution is larger than or equal to 80 g / L, the main impurity contents are all smaller than or equal to 10 ppm, the heavy metal content is smaller than or equal to 1 ppm, and the short-process low-consumption free-pollution high-purity manganese sulfate solution preparing method is provided.
Owner:UNIV OF SCI & TECH BEIJING
Method for continuously preparing hydrogen sulfide and coproducing nano barium sulfate through microreactor
PendingCN109052449AIncrease profitImprove qualityCalcium/strontium/barium sulfatesHydrogen sulfidesMicroreactorSulfate
The invention discloses a method for continuously preparing hydrogen sulfide and coproducing nano barium sulfate through a microreactor. The method comprises the following steps: dissolving barium sulfide in a dissolving tank at the temperature of 60-90 DEG C; filtering, and preparing a solution from desalted water to obtain a 0.5-2mol / L barium sulfide solution; storing into a storing tank A; preparing a 0.5-2mol / L sulfuric acid solution from industrial sulfuric acid in a storing tank B; pumping the barium sulfide solution in the storing tank A and the sulfuric acid solution in the storing tank B into the microreactor based on the molar ratio of 1: (1-1.1); and reacting at the temperature of 60-90 DEG C to obtain barium sulfide slurry and hydrogen sulfide; collecting the overflowing hydrogen sulfide and storing in a buffering gas tank; filtering the barium sulfide slurry; washing and separating with the desalted water; and performing spray drying at the temperature of 110-150 DEG C toobtain the nanobarium sulfate powder. With the adoption of the method, the hydrogen sulfide can be continuously produced; the cost is low; and the quality is stable.
Owner:GUIZHOU MICRO CHEM TECH CO LTD
Heavy metal-containing wastewater treatment chemical
InactiveCN104402070AUniversalEasy to handleWater contaminantsWater/sewage treatment by flocculation/precipitationPapermakingWater quality
The invention relates to a heavy metal-containing wastewater treatment chemical. The wastewater treatment chemical is prepared by mixing barium sulfide, ferric sulfate, poly-silicon aluminum sulfate and a few calcium hydroxide and lithopone, wherein barium sulfide accounts for 30-40 parts by weight, ferric sulfate accounts for 20-30 parts by weight, poly-silicon aluminum sulfate accounts for 20-40 parts by weight, calcium hydroxide accounts for 5 parts by weight and lithopone accounts for 5 parts by weight. The chemical has a wide range of application and has high universality. The wastewater treatment chemical has a good effect of treating industrial wastewater of metallurgy, heavy metal, mine, printing and dyeing, spinning, building, casting, chemical, electric power, leather and papermaking, etc. as well as municipal composite wastewater. The chemical has a wide range of pH for water and has purifying effects at pH 4-10. The chemical has good sedimentation efficiency and good water purification quality and is non-corrosive. The pH value of a solution treated by the use of the chemical is 6.5-8.5 and is close to neutral. The solution contains no chloride ion. A steel structure in a water system can effectively be protected during recycling so as to protect the steel structure from being corroded. Thus, a large amount of equipment maintenance cost can be minimized annually.
Owner:WUXI EPIC TECH
Method for preparing silica modified barium sulfate powder
ActiveCN107746068AHigh activitySmall sizeMaterial nanotechnologyCalcium/strontium/barium sulfatesBarium sulfideSodium silicate
A method for preparing silica modified barium sulfate powder comprises the following steps: adding a barium sulfide solution into a sodium carbonate solution, performing stirring reacting at 20-95 DEGC for 0.5-4 h, filtering the obtained solution, adding the obtained filter cake into a sodium sulfate solution, performing high-shearing stirring reacting at 20-95 DEG C for 0.5-8 h, adding sodium silicate accounting for 0.1-10% of the mole of the barium sulfide, adding a sulfuric acid solution according to a molar ratio of sulfuric acid to the sodium silicate being (1.1-1.2):1, continuously performing stirring reacting for 1-2 h, and sequentially carrying out filtration, water washing and 100-200 DEG C drying to obtain the silica modified barium sulfate. The method has the advantages of simple process, less device investment, and realization of large-scale industrialization.
Owner:CHINA RES INST OF DAILY CHEM IND
Composite water treatment agent and preparation method thereof
InactiveCN106587393AEasy to handleExcellent corrosion and scale inhibitionScale removal and water softeningIron sulfateSlag
Owner:张伟荃
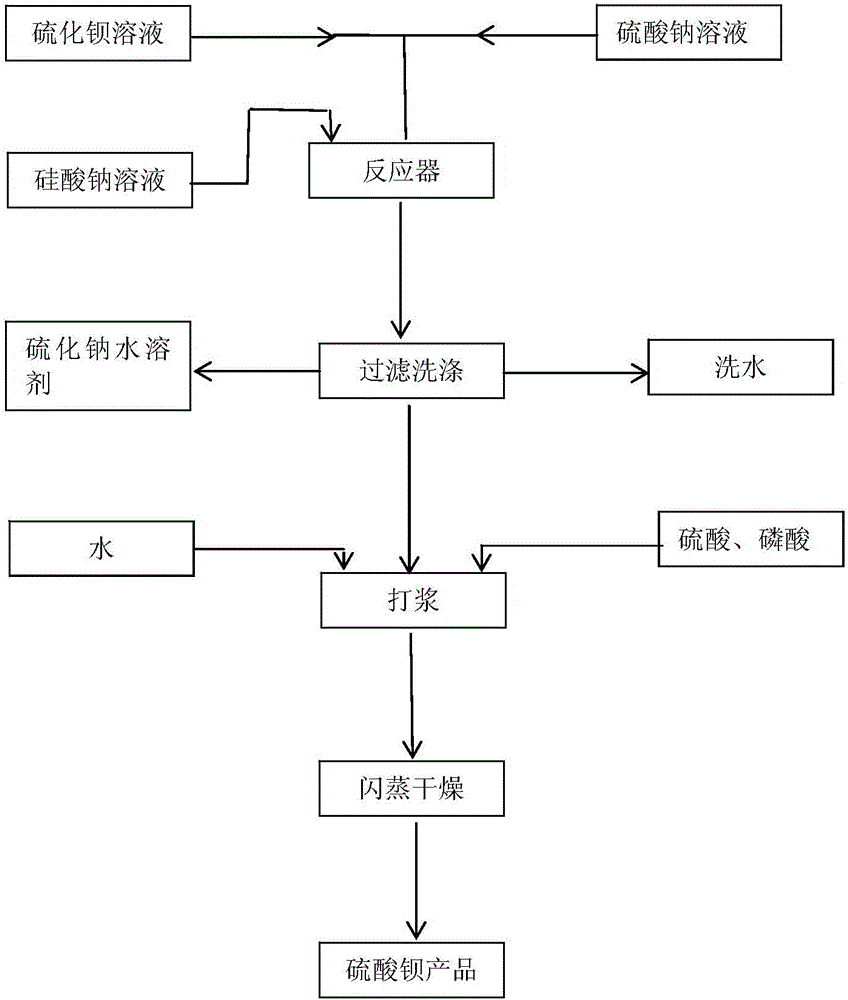
Production method of micro-particle size precipitated barium sulfate
InactiveCN106186027AHigh glossGood biocompatibilityCalcium/strontium/barium sulfatesSulfurPhosphoric acid
The invention discloses a production method of micro-particle size precipitated barium sulfate. The production method comprises the following steps: (S100) dissolving roughed barium sulfide black ash into water, so as to prepare a clear barium sulfide solution with a concentration of 190g / L-200g / L and a temperature higher than or equal to 85 DEG C for later use; (S200) preparing a clear sodium sulfate solution with a concentration of 280g / L-300g / L and a temperature higher than or equal to 85 DEG C from sodium sulfate for later use; (S300) preparing a sodium silicate solution for later use, wherein the addition amount of the sodium silicate solution is 1g / m<3>-10g / m<3>; (S400) mixing the prepared barium sulfide solution with the prepared sodium sulfate solution to react in a reactor, stirring at a speed of 15rov / min-25rov / min to control the uniformity of the reaction, and when the barium content in the solution is slightly large, adding the sodium silicate solution to react for 1h-1.5h so as to reach an equivalent point; (S500) filtering the solution after the reaction, washing until no sulfur ion exists, pulping a barium cake with water, and regulating the pH value to 6.5-7.5 with concentrated sulfuric acid or phosphoric acid; and (S600) filtering barium pulp obtained in the step (500), and carrying out expansion drying at 200-300 DEG C. Barium sulfate prepared by virtue of the production method is high in purity and small in particle size and can be widely applied.
Owner:SHENZHEN JIAXIN CHEM CO LTD
Paint containing photo-induced energy-storage luminous powder
InactiveCN105419638AStrong covering powerGood gloss retentionLuminescent paintsEpoxy resin coatingsEpoxyPolyvinyl alcohol
The invention relates to a chemical industrial product, and especially relates to a paint containing photo-induced energy-storage luminous powder. The paint containing the photo-induced energy-storage luminous powder comprises the following components by weight ratio: 5-5.5% of barium sulfide, 3-4% of magnesium sulfide, 1.5-2.2% of aluminum sulfide, 2-3% of potassium nitrate, 20-30% of tung oil, 10-16% of epoxy resin, 5-11% of acrylonitrile, 3-6% of modified alkyd resin, 5-8% of methacrylic resin, 0.5-1% of turpentine, 1-3% of a propylene pigment, 3-6% of a photoinitiator, 5-10% of polyvinyl butyral, 3-5% of an antifoaming agent and the balance of a thinner. The paint containing the photo-induced energy-storage luminous powder has the advantages of strong covering power, good gloss retention, color retention and weatherability, and fast drying speed; can absorb yellow light emitted under dark environment, and can be used for objects such as guideboards, outdoor advertisements, night roads and obstacle marks.
Owner:QINGDAO CHAOYANG HUATAI MANAGEMENT CONSULTATION SERVICE CO LTD
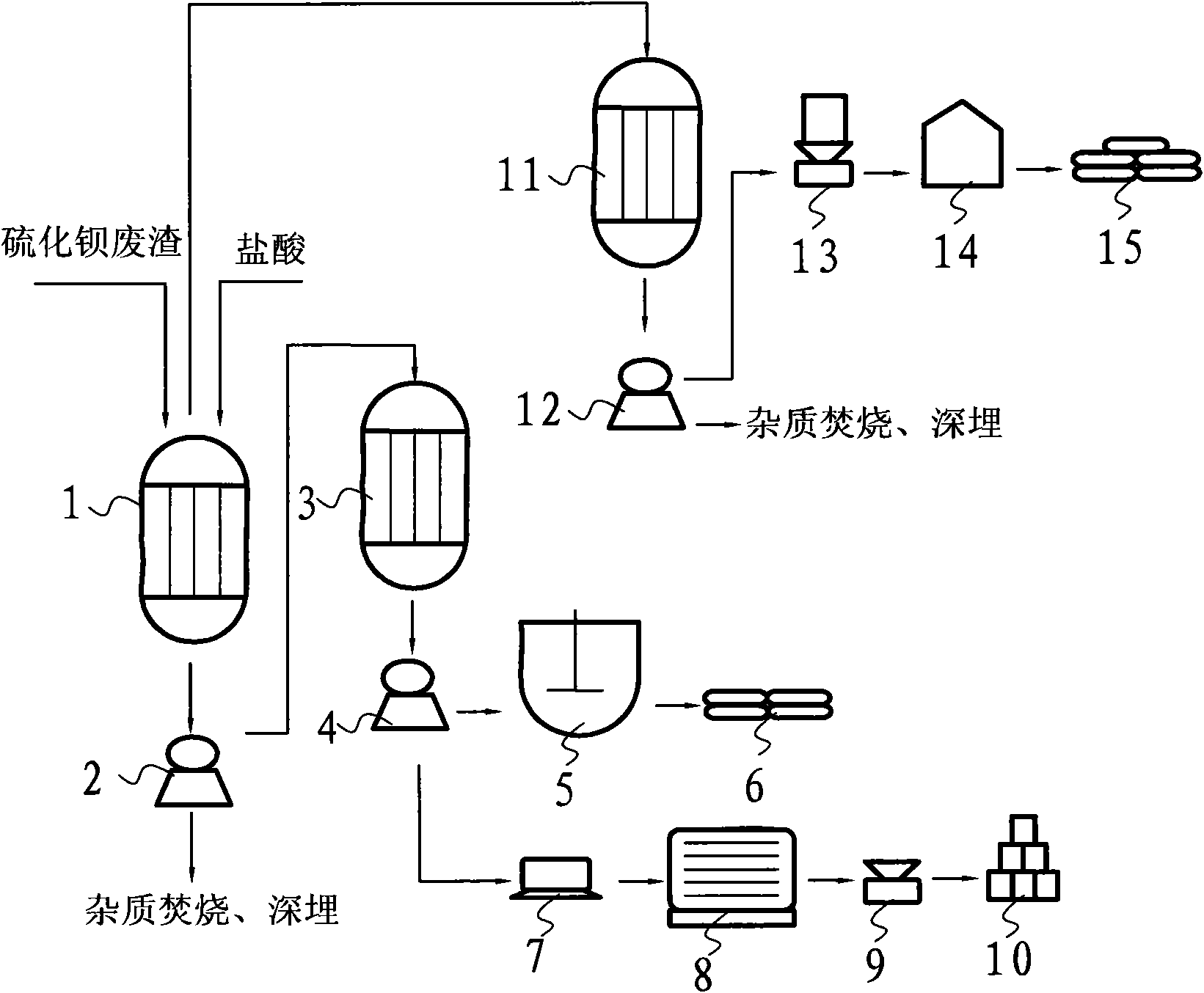
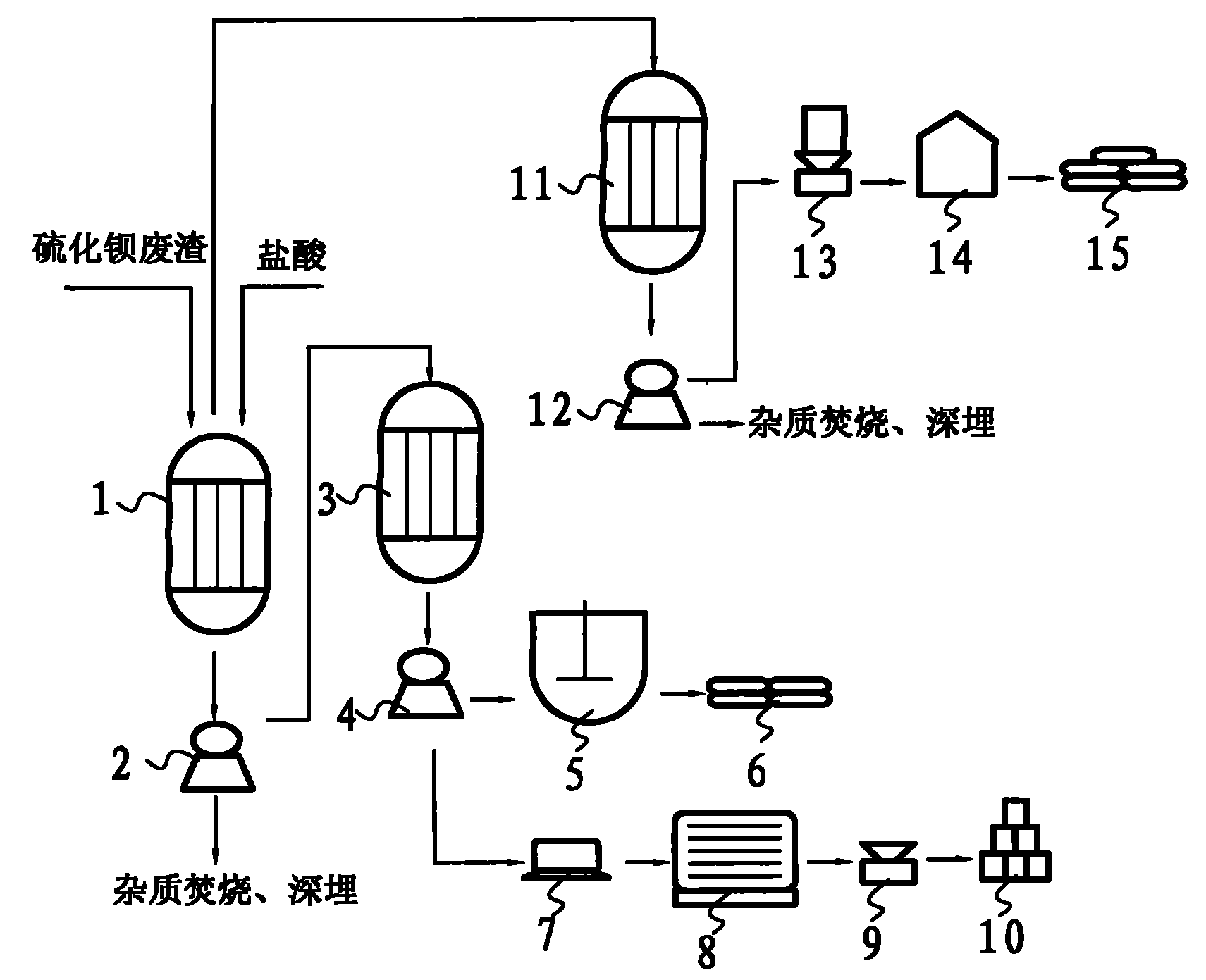
Method for coproducing sodium sulphide and industrial salts while preparing precipitated barium sulfate from barium sulfide waste slag
InactiveCN101987741APollution controlHigh economic valueSolid waste disposalCalcium/strontium/barium sulfatesSlagSodium sulfate
The invention belongs to the technical field of chemical and industrial three-waste disposal and utilization, in particular to a method for coproducing sodium sulphide and industrial salts while preparing precipitated barium sulfate from barium sulfide waste slag. In the main technical scheme of the invention, hydrochloric acid is mainly and fully used for acidification to completely remove insoluble impurities so as to obtain purer solutions such as barium chloride and the like, then, sodium sulfate is used for exchange to obtain precipitated barium sulfate and byproducts of the sodium sulphide and the industrial salts, therefore, by using the invention, a large amount of barium sulfide waste slag is eliminated when products with higher economic value are prepared. The method of the invention has the advantages of low production cost, high profit, large market demand and simple process and production equipment, belongs to a very practical treatment and utilization method, not only can create considerable economic value, but also can eliminate the chemical and industrial waste slag and control the environment pollution, particularly has thoroughly control results on the barium sulfide waste slag, and avoids secondary pollution and hidden trouble in environment protection.
Owner:汪晋强
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com