Method for coproducing sodium sulphide and industrial salts while preparing precipitated barium sulfate from barium sulfide waste slag
A technology for precipitating barium sulfate and barium sulfide, which is applied to the removal of calcium/strontium/barium sulfate, alkali metal sulfide/polysulfide, and solid waste, and can solve problems such as occupying large land, waste, and environmental pollution. , achieve the effect of high profit, low cost, and environmental pollution control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
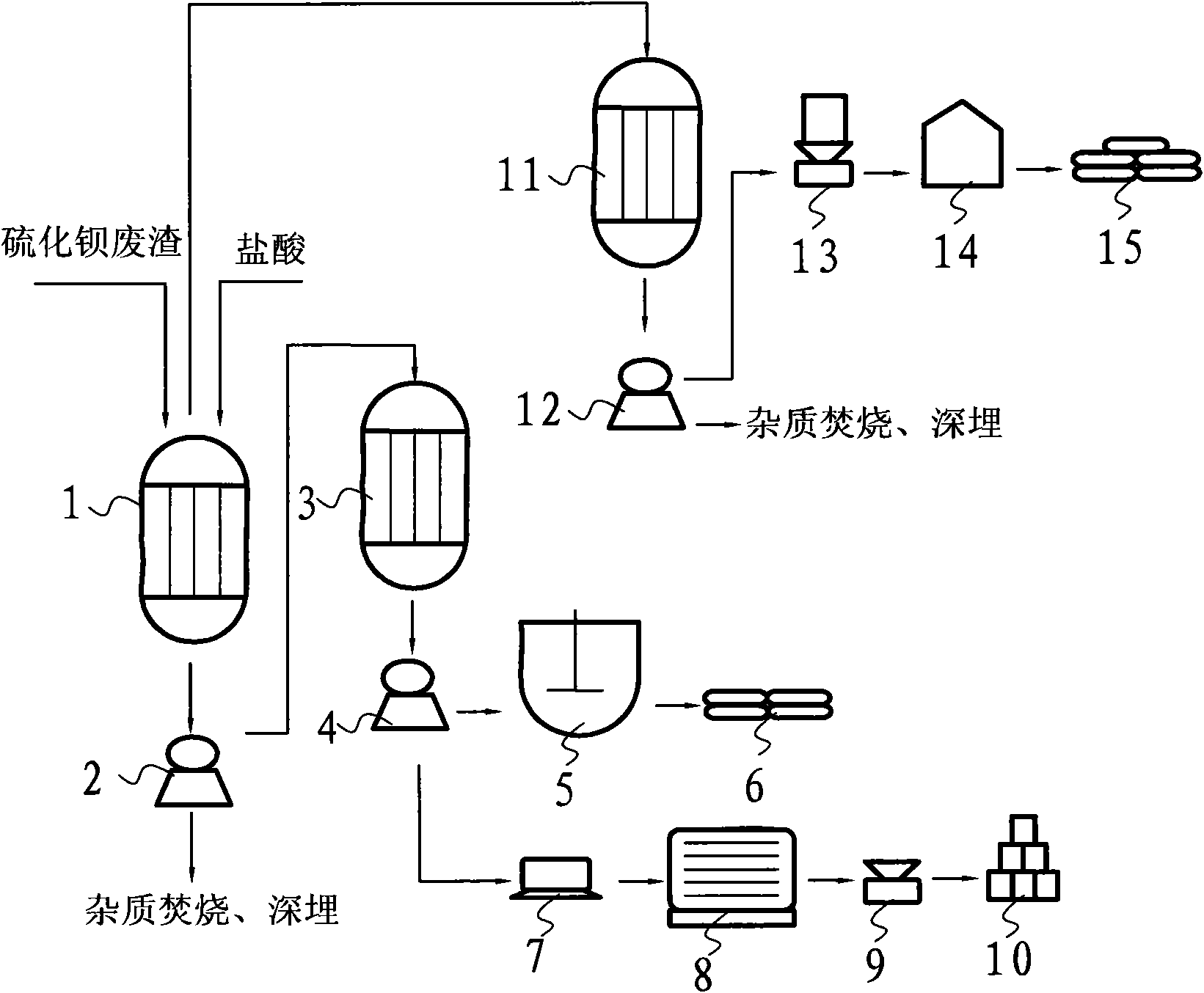
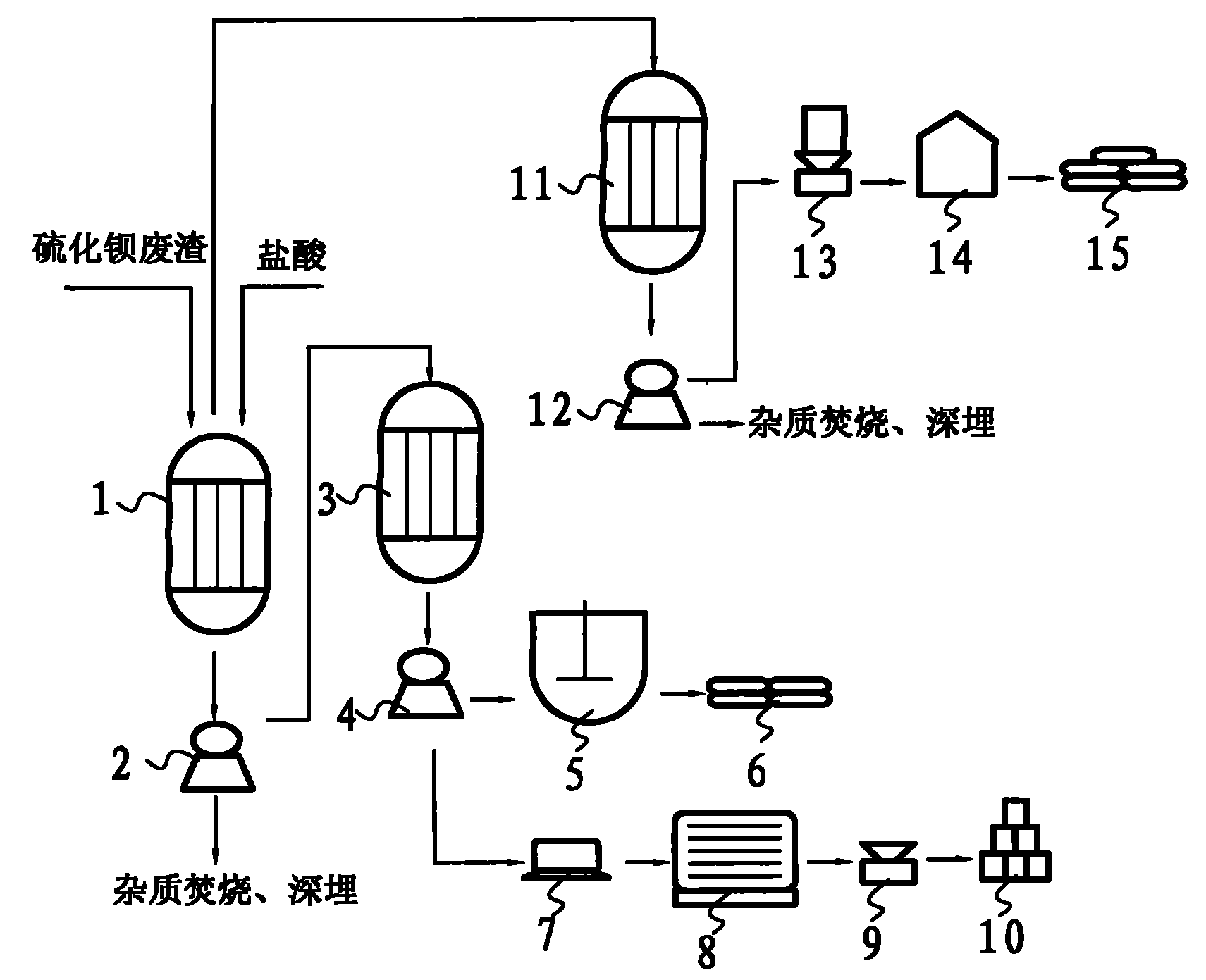
[0024] A method for preparing precipitated barium sulfate to co-produce soda sulfide and industrial salt with barium sulfide waste residue. The converted barium sulfide waste residue (calculated as 400kg barium sulfide) and hydrochloric acid are sequentially put into the anti-corrosion reactor with a pure mass ratio of 1:0.26 One mile, chemical reaction is carried out under slow stirring;
[0025] The reaction mixture that reaction obtains is filtered with filter one 2, removes insoluble impurity, and barium chloride filtrate and sodium sulfate drop into anticorrosion reactor two 3 lis successively with pure mass ratio 1: 0.52 and carry out stirring reaction (the described filtrate and calcium hydroxide The pure mass ratio is converted according to pure barium chloride in the filtrate), then filter with filter 2 4, and the sodium chloride filtrate obtained by filtering is distilled with distiller 1 5 to obtain 333.83kg industrial salt 6; Barium sulfate filter cake washes, drie...
Embodiment 2
[0028] A method for preparing precipitated barium sulfate to co-produce soda sulfide and industrial salt with barium sulfide waste residue. The converted barium sulfide waste residue (calculated as 400kg barium sulfide) and hydrochloric acid are sequentially put into the anti-corrosion reactor with a pure mass ratio of 1:0.46 One mile, chemical reaction is carried out under slow stirring;
[0029] The reaction mixture that reaction obtains is filtered with filter one 2, removes insoluble impurity, and barium chloride filtrate and sodium sulfate drop into anticorrosion reactor two 3 li successively with pure mass ratio 1: 0.72 and carry out stirring reaction (the filtrate and calcium hydroxide The pure mass ratio is converted according to pure barium chloride in the filtrate), then filter with filter 2 4, and the sodium chloride filtrate obtained by filtering obtains 590.62kg industrial salt 6 with distiller 1 5 distillation; Barium sulfate filter cake washes, dries and pulveri...
Embodiment 3
[0032] A method for preparing precipitated barium sulfate to co-produce soda sulfide and industrial salt with barium sulfide waste residue. The converted barium sulfide waste residue (calculated as 400kg barium sulfide) and hydrochloric acid are sequentially put into the anti-corrosion reactor with a pure mass ratio of 1:0.66 One mile, chemical reaction is carried out under slow stirring;
[0033] The reaction mixture that reaction obtains is filtered with filter one 2, removes insoluble impurity, and barium chloride filtrate and sodium sulfate drop into anticorrosion reactor two 3 li successively with pure mass ratio 1: 0.92 and carry out stirring reaction (the filtrate and calcium hydroxide The pure mass ratio is converted according to pure barium chloride in the filtrate), then filter with filter 2 4, and the sodium chloride filtrate obtained by filtering obtains 847.41kg industrial salt 6 with distiller 1 5 distillation; Barium sulfate filter cake washes, dries and pulveri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com