Barium carbonate, preparation method thereof and carbonizer
A technology of barium carbonate and carbonization tower, which is applied in the field of high-reactivity barium carbonate and its preparation, can solve the problems of low reactivity of barium carbonate powder, and achieve slowing down of crystal development and growth, uniform particle size distribution, and increased reaction nucleation speed Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
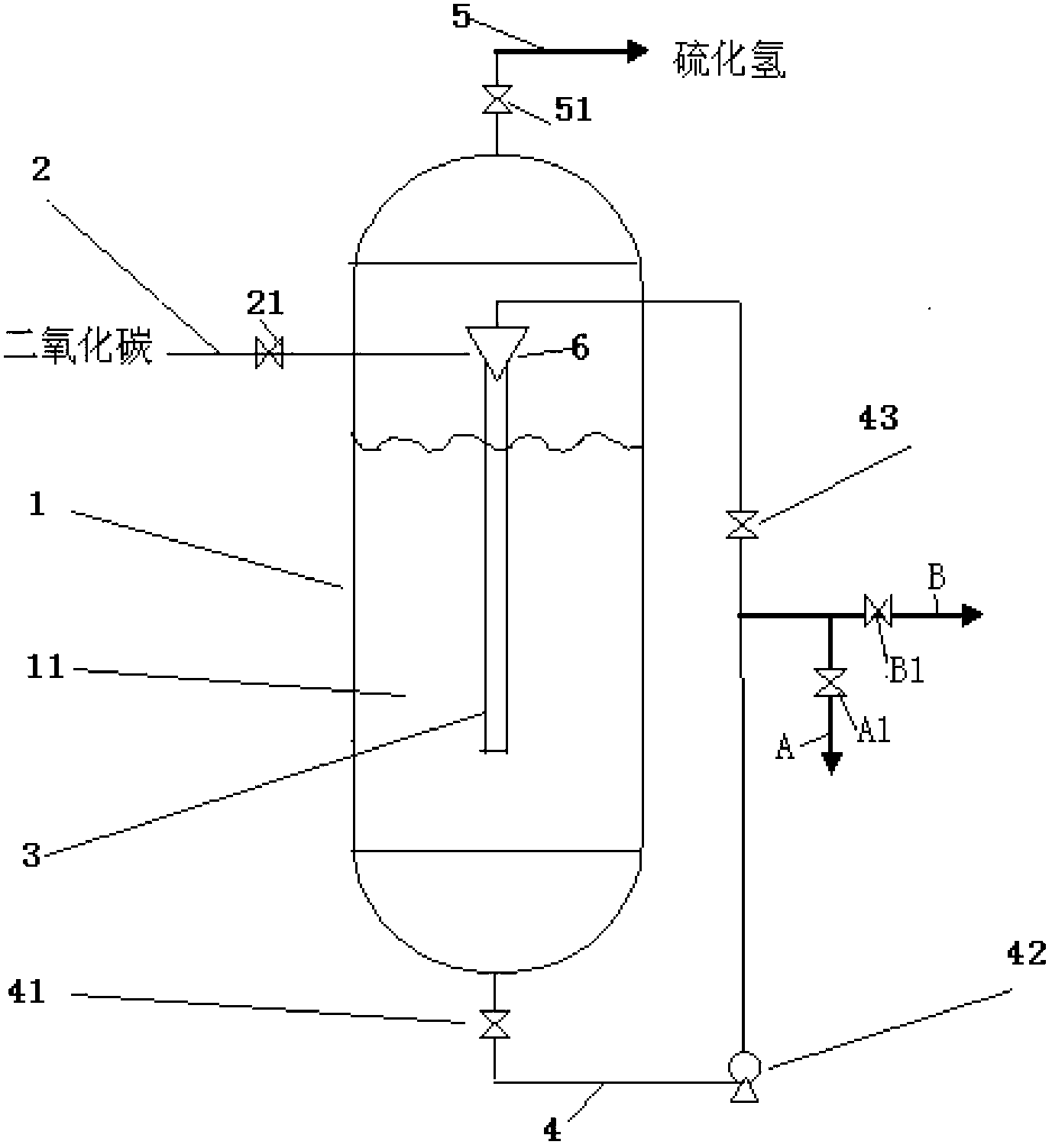
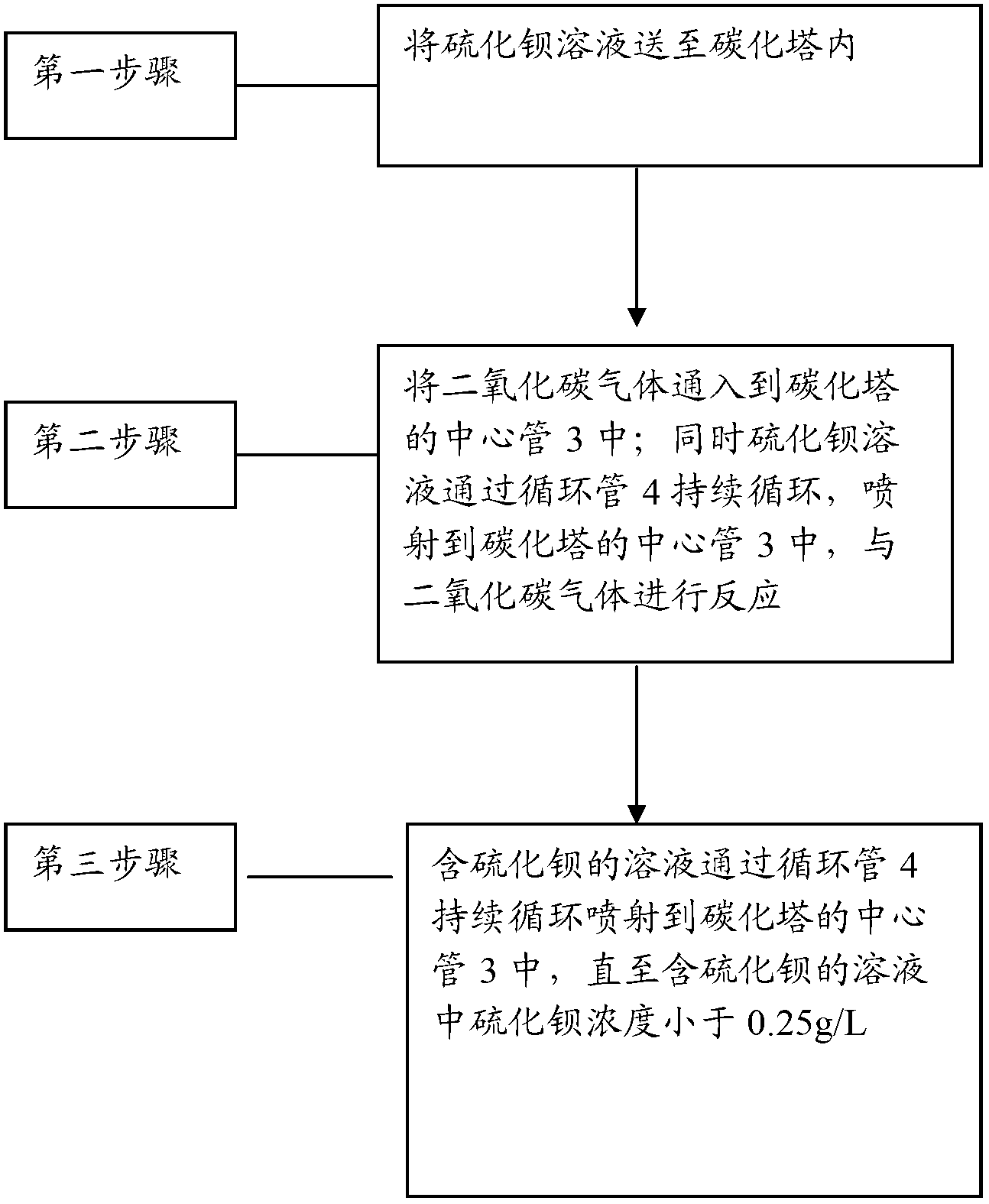
[0027] Below in conjunction with carbonization tower, describe the preparation method of barium carbonate of the present invention in detail. The method includes:
[0028] The first step is to prepare a barium sulfide solution with a concentration of 120-170g / L (preferably 120-140g / L), and maintain its temperature at 65-75°C;
[0029] In the second step, the barium sulfide solution is sent to the cavity 11 of the carbonization tower, so that the barium sulfide solution accounts for 50-70% (preferably 60%) of the volume of the cavity 11;
[0030] In the third step, the carbon dioxide gas is passed into the center pipe 3; at the same time, the barium sulfide solution circulates through the circulation pipe 4, flows into the circulation pipe 4 from the chamber 11, and is then sprayed into the center pipe 3 by the injector 6, and is carried out with the carbon dioxide gas. reaction;
[0031] In the fourth step, the solution containing barium sulfide continues to circulate throug...
Embodiment 1
[0046] The carbonization tower used in this embodiment is made of corrosion-resistant stainless steel. The cavity 11 is a cylinder with a diameter of 2m×a height of 3m. The central tube 3 is DN with a diameter of 150mm and a length of 2m.
[0047] A barium sulfide solution with a concentration of 120 g / L was prepared and its temperature was maintained at 65°C. The barium sulfide solution is routinely pumped into the cavity 11 of the carbonization tower, so that the barium sulfide solution accounts for 50% of the volume of the cavity 11, and the lower end of the carbonization tower central tube 3 is located under the barium sulfide solution liquid level. Open the valve 21, the valve 41, the valve 43, and the valve 51, and the carbon dioxide gas enters the center pipe 3 continuously through the intake pipe 2, and the flow rate in the center pipe 3 is 1400Nm 3 / h. At the same time, the barium sulfide solution in the carbonization tower continuously circulates through the circula...
Embodiment 2
[0050] The carbonization tower used in this embodiment is made of corrosion-resistant stainless steel. The cavity 11 is a cylinder with a diameter of 2m×a height of 3m. The central tube 3 is DN with a diameter of 120mm and a length of 2m.
[0051] A barium sulfide solution with a concentration of 140 g / L was prepared and its temperature was maintained at 65°C. The barium sulfide solution is routinely pumped into the cavity 11 of the carbonization tower, so that the barium sulfide solution accounts for 60% of the volume of the cavity 11, and the lower end of the carbonization tower central tube 3 is located under the barium sulfide solution liquid level. Open the valve 21, the valve 41, the valve 43, and the valve 51, and the carbon dioxide gas enters the center pipe 3 continuously through the intake pipe 2, and the flow rate in the center pipe 3 is 1600Nm 3 / h. At the same time, the barium sulfide solution in the carbonization tower continuously circulates through the circula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com