Short-process low-consumption high-purity manganese sulfate solution preparing method
A manganese sulfate solution and low-consumption technology, applied in the direction of manganese sulfate, etc., can solve the problems of removing calcium and magnesium impurities, manganese loss, high content, and difficulty in removing, and achieve the effects of reducing production costs, shortening the process flow, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
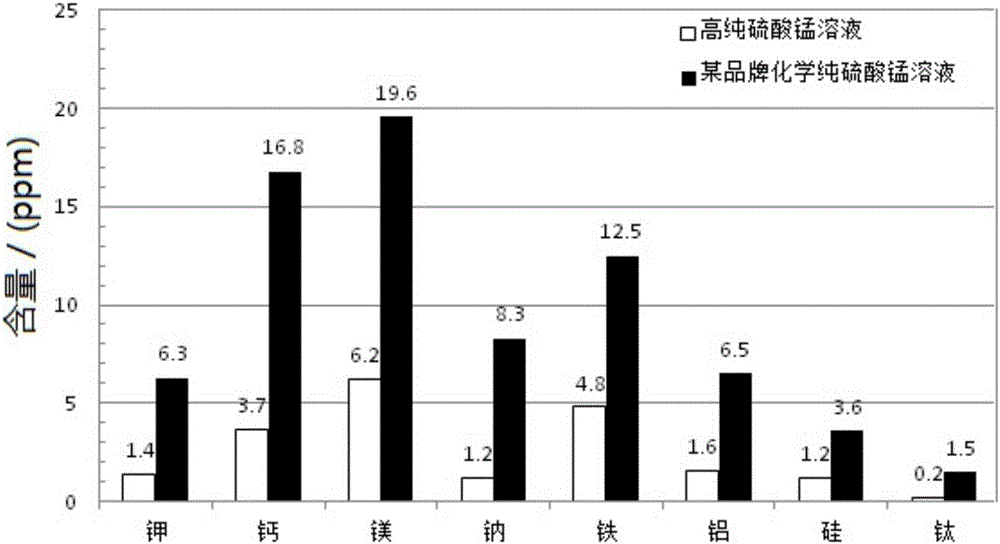
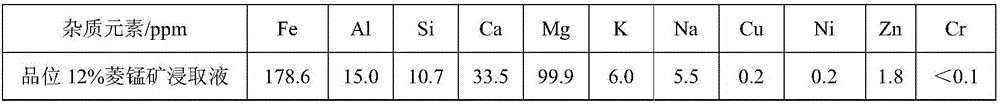
[0019] (1) The rhodochrosite of grade 12% is ground, crosses 200 mesh sieves, obtains manganese ore powder; Manganese ore powder is joined in the sulfuric acid solution of 2mol / L, and the mass ratio of sulfuric acid solution and manganese ore powder is 3:1, stirs After being heated to 90°C under certain conditions, the reaction was continued for 4 hours, and the manganese ore leaching solution 1 was obtained by filtration, and the impurity content was analyzed by ICP;
[0020] (2) Add manganese ore powder to manganese ore leaching solution 1, the mass ratio of manganese ore leaching solution 1 to manganese ore powder is 3:1, heat to 90°C under agitation and continue to react for 4 hours, and filter to obtain manganese ore leaching solution 2 ;
[0021] (3) Regulate the pH of manganese ore leaching solution 2 to 4 with barium hydroxide, then add barium fluoride until no new precipitation occurs, then filter to obtain filtrate 1, wherein barium hydroxide is 100 mesh sieves with ...
Embodiment 2
[0029] (1) grind the rhodochrosite of grade 14%, cross 150 mesh sieves, obtain manganese ore powder; Manganese ore powder is joined in the sulfuric acid solution of 1.8mol / L, the mass ratio of sulfuric acid solution and manganese ore powder is 4:1, in Continue to react for 3 hours after heating to 85°C under stirring conditions, filter to obtain manganese ore leaching solution 1, and use ICP to analyze its impurity content;
[0030] (2) Add manganese ore powder to the manganese ore leaching solution 1, the mass ratio of the manganese ore leaching solution 1 and the manganese ore powder is 5:1, heat to 80° C. under agitation and continue to react for 3 hours, and filter to obtain the manganese ore leaching solution 2;
[0031] (3) Regulate the pH of manganese ore leaching solution 2 to 3 with barium hydroxide, add barium fluoride again, until no new precipitation produces, then filter, obtain filtrate 1, wherein barium hydroxide is that content 98% crosses 100 mesh sieves Bariu...
Embodiment 3
[0038] (1) The rhodochrosite of grade 11% is ground, crosses 100 mesh sieves, obtains manganese ore powder; Manganese ore powder is joined in the sulfuric acid solution of 1.5mol / L, and the mass ratio of sulfuric acid solution and manganese ore powder is 5:1, in Continue to react for 2 hours after heating to 80°C with stirring, and filter to obtain manganese ore leaching solution 1;
[0039](2) Add manganese ore powder to manganese ore leaching solution 1, the mass ratio of manganese ore leaching solution 1 and manganese ore powder is 4:1; continue to react for 2 hours after heating to 85°C under stirring conditions, and filter to obtain manganese ore leaching solution 2 ;
[0040] (3) Regulate the pH of manganese ore leaching solution 2 to 3 with barium hydroxide, add barium fluoride again, until no new precipitation produces, then filter, obtain filtrate 1, wherein barium hydroxide is that content 98% crosses 100 mesh sieves Barium hydroxide octahydrate solid powder, barium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com