Method for preparing precipitated barium sulfate by utilizing titanium white waste acid
A technology for precipitating barium sulfate and titanium white waste acid, applied to chemical instruments and methods, calcium/strontium/barium sulfate, titanium compounds, etc., can solve the problems of many steps, cumbersome impurity removal process, and long processing time, and achieve Good economy, reduce processing cost and environmental protection pressure, and save resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
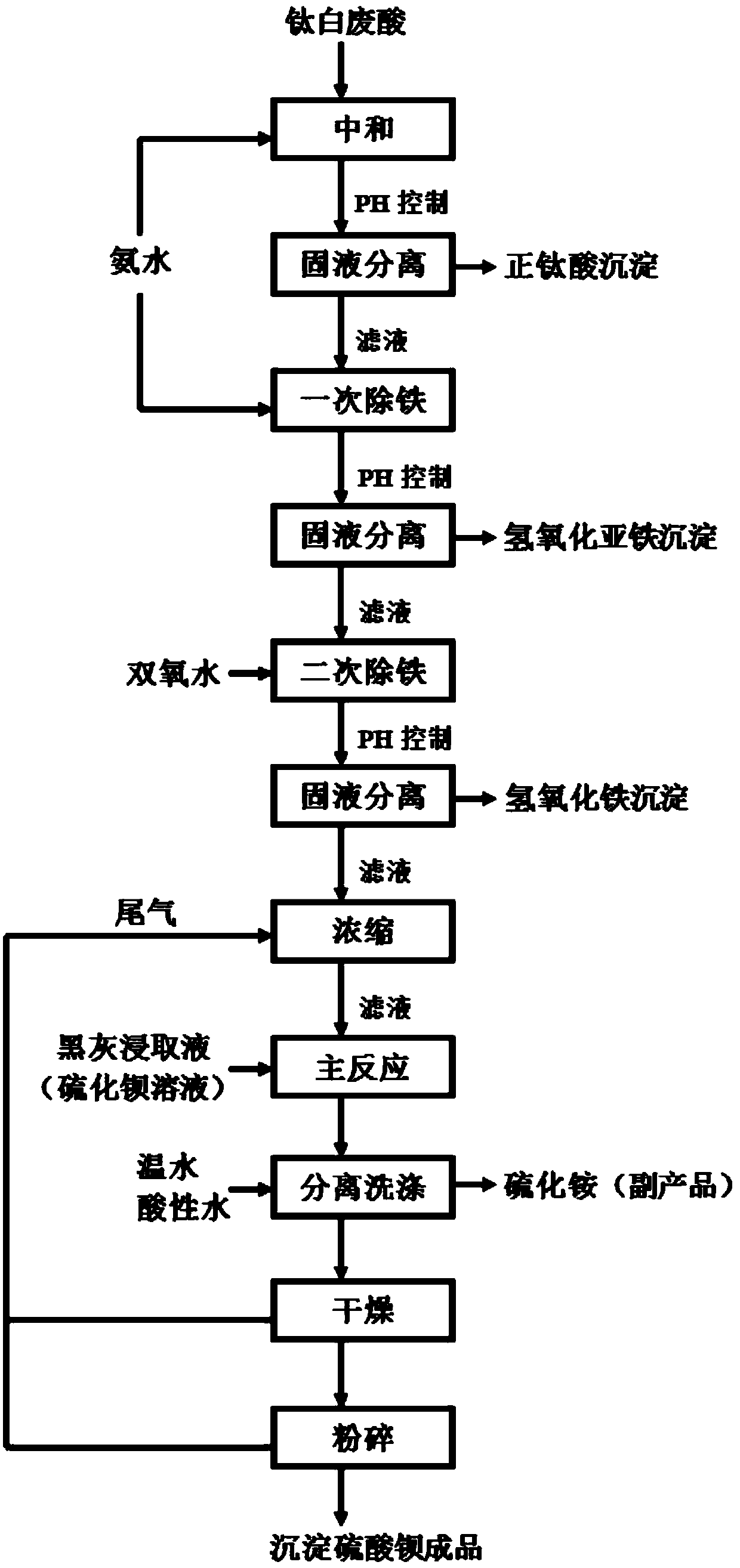
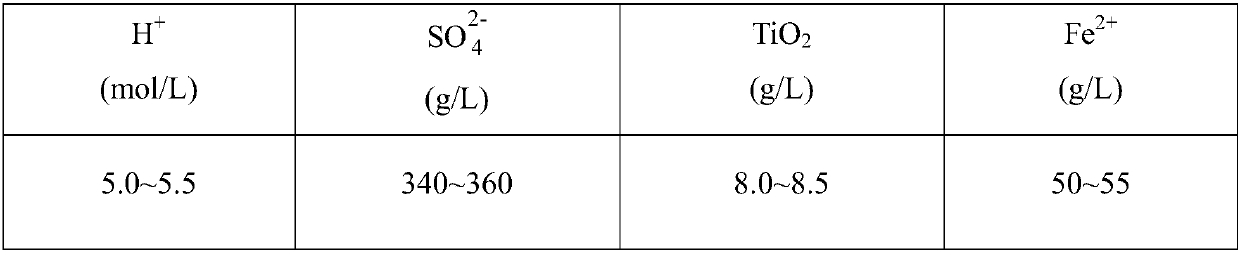
[0058] Step 1. Transfer a certain amount of titanium white waste acid from the waste acid storage tank to the neutralization tank, start stirring, and evenly add ammonia water to the neutralization tank through the ammonia water storage tank to neutralize the titanium white waste acid to generate ammonium sulfate solution. According to the online pH According to the detection data of the probe, the pH of the neutralization tank system is controlled at 3.0, and the addition of ammonia water is stopped, and the concentration of the ammonia solution is 25%.
[0059] Step 2: Stir the neutralized solution and precipitate in the neutralization tank for 20 minutes to make titanyl sulfate generate orthotitanic acid precipitation reaction fully, then pump it to the frame filter press for filtration, and the filtrate from the frame filter press The filtrate exits into the primary iron removal tank, and the ortho-titanic acid filter cake can be used for titanium dioxide production after c...
Embodiment 2
[0069] Step 1. Transfer a certain amount of titanium white waste acid from the waste acid storage tank to the neutralization tank, start stirring, and add ammonia water to the neutralization tank through the ammonia water storage tank at a molar ratio of 2:1 to neutralize the titanium white waste acid to generate sulfuric acid Ammonium solution, according to the detection data of the online pH probe, control the pH of the neutralization tank system at about 3.0, stop adding ammonia water, the concentration of the ammonia solution is 27%, at this time, the titanyl sulfate in the titanium white waste acid produces orthotitanic acid precipitation;
[0070] Step 2: Stir the neutralized solution and precipitate in the neutralization tank for 30 minutes to make titanyl sulfate generate orthotitanic acid precipitation reaction fully, then pump it to the frame filter press for filtration, and the filtrate from the frame filter press The filtrate exits into the primary iron removal tank...
Embodiment 3
[0080] Step 1. Transfer a certain amount of titanium white waste acid from the waste acid storage tank to the neutralization tank, start stirring, and add ammonia water to the neutralization tank through the ammonia water storage tank at a molar ratio of 2:1 to neutralize the titanium white waste acid to generate sulfuric acid Ammonium solution, according to the detection data of the online pH probe, control the pH of the neutralization tank system at about 3.0, stop adding ammonia water, the concentration of the ammonia solution is 28%, at this time, the titanyl sulfate in the titanium dioxide waste acid produces orthotitanic acid precipitation;
[0081] Step 2: Stir the neutralized solution and the precipitate in the neutralization tank for 40 minutes to make titanium oxysulfate generate orthotitanic acid precipitation reaction fully, then pump it to the frame filter press for filtration, and the filtrate from the frame filter press The filtrate exits into the primary iron re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com