Patents
Literature
127 results about "Iron(II) hydroxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Iron(II) hydroxide or ferrous hydroxide is an inorganic compound with the formula Fe(OH)₂. It is produced when iron(II) salts, from a compound such as iron(II) sulfate, are treated with hydroxide ions. Iron(II) hydroxide is a white solid, but even traces of oxygen impart a greenish tinge. The air-oxidized solid is sometimes known as "green rust".
Method for producing iron oxide black
This is the producing method of a kind of oxidized iron oxide black including following contents: prepare ferrite watery solution; use sodium hydroxide as neutralizaing agent and react with ferrite watery solution to produce hydroxide ferrous; use oxygenous gas as oxidant, and react with said hydroxide ferrous to produce iron oxide black; make the prepared iron oxide black water washed, sieved, dried and comminuted orderly to get blue oxidized iron oxide black pigment product. The oxidized iron oxide black produced by this method has high purity and thin crystal grain, the grain size of produced pigment is thin and even, and has saturated blue ink light black and stable effect to light and atmosphere. It also has strong pigmentation strength and hiding power, and can be used in such fine chemical industry products as printing ink and carbon powder. The farther project is using waste residue containing ferrous sulfate and vented in titanium oxide powder production, to produce ferrous sulfate watery solution and produce oxidized iron oxide black, which can adequately use industry waste material to produce oxidized iron oxide black products of high quality, and reduce environmental pollution.
Owner:HUBEI WANRUN NEW ENERGY TECH DEV
Process and method for the removal of arsenic from water
This invention describes a one step process for the removal of heavy metals, particularly arsenic, from water. The process consists in promoting the circulation of the water to be treated in an electrolytic cell equipped with iron, or iron alloy anodes and cathodes made of iron or iron alloy or other metals, while the contemporary insufflation into the cell of a gas, partially or totally composed of oxygen. In this way the iron of the anode electrodes dissolves as iron hydroxide. The ferrous hydroxide thus generated, under the action of the oxygen contained in the insufflated gas is converted to ferric hydroxide, which, through a complex mechanism, adsorbs and forms insoluble complexes with the arsenic ions. At the same time As(III) is subject to oxidation both at the anode and at the cathode. By this process both forms of arsenic, As(III) and As(V), are equally removed. The treated water is further processed by conventional clarifying and filtering.
Owner:DEL SIGNORE GIOVANNI
Method for preparing ferriferrous oxide black pigment
InactiveCN1807262AReduce restrictionsWide spectrumPigmenting treatmentFerroso-ferric oxidesBlack paintPigment
The invention discloses a preparation method for Fe3O4 as black paint, which comprises preparing the FeSO4 solution, Fe(OH)2 and Fe3O4 by turns. With USA datacolor color photometer, CIE standard and D65 standard light source, the YP / TD / S-01 testing shows the product comes up to the index completely. Compared with prior art, this invention has short production period and small limit to material; the product has wide product color spectrum range to meet wide market application request with low cost.
Owner:SHANGHAI YIPIN PIGMENTS CO LTD
Method for preparing magnetic ferric oxide nano particles
The invention discloses a preparation method for a magnetic iron oxide nanometer particle. The cleaned cellulose fibers are immersed in the 0.1-0.5mol / l ferric salt or ferrous salt solution; after the ferric ions in the ferric salt or ferrous salt solution are fully absorbed in the micro-pores of the cellulose fibers or on the surface of the cellulose fibers, the cellulose fibers are immersed in the 0.2-4mol / l NaOH solution; the ferric ions and the ferrous ions in the solution generate the ferric hydroxide precipitation or the ferrous hydroxide precipitation in the micro-pores of the cellulose fibers to constitute the cellulose composite fibers; the ferric oxide / cellulose composite fibers are obtained after the dehydration of the cellulose composite fibers; finally, the ferric oxide / cellulose composite fibers are ignited in the air atmosphere or the oxygen atmosphere at the temperature of 350-1000 Celsius system for 20-300 minutes, so that the magnetic iron oxide nanometer particles with high purity are obtained. The method has a simple operation and the low cost raw materials, the prepared iron oxide nanometer particles have the even diameter distribution with a certain magnetism.
Owner:WUHAN UNIV
Method for producing iron oxide black
ActiveCN1709984AHigh tinting strengthUniform particle sizePigmenting treatmentReaction rateIron Black
The invention relates to the producing method of a kind of oxidized iron black. It includes following steps: add ferrite watery solution into neutralization action pot, add ammonia and mix round watery solution to neutralize it to prepare hydroxidized ferrous gelatin, transmit the produced gelatin into oxidizing reaction pot; send oxygenous gas and meanwhile heat gelatin to make it oxidated, after reaction we can get oxidized iron oxide black pulp; separate iron oxide black and mother liquor in pulp; make the separated iron oxide black water washed, sieved, dried and comminuted orderly to get finished products. This method adopts liquid phase method to make its reaction rate greatly improved, the particle granularity of oxidized iron oxide black finished products even; it has higher pigmentation strength, and the obtained product contains 96.5% Fe3O4. Compared with the industry appointed standard sample, its pigmentation strength is 200% - 250%; ulteriorly make offal in producing titanium oxide powder as material of this method, which is beneficial to circulation usage of industry waste material and environmental protection, and can greatly reduce producing cost.
Owner:HUBEI WANRUN NEW ENERGY TECH DEV
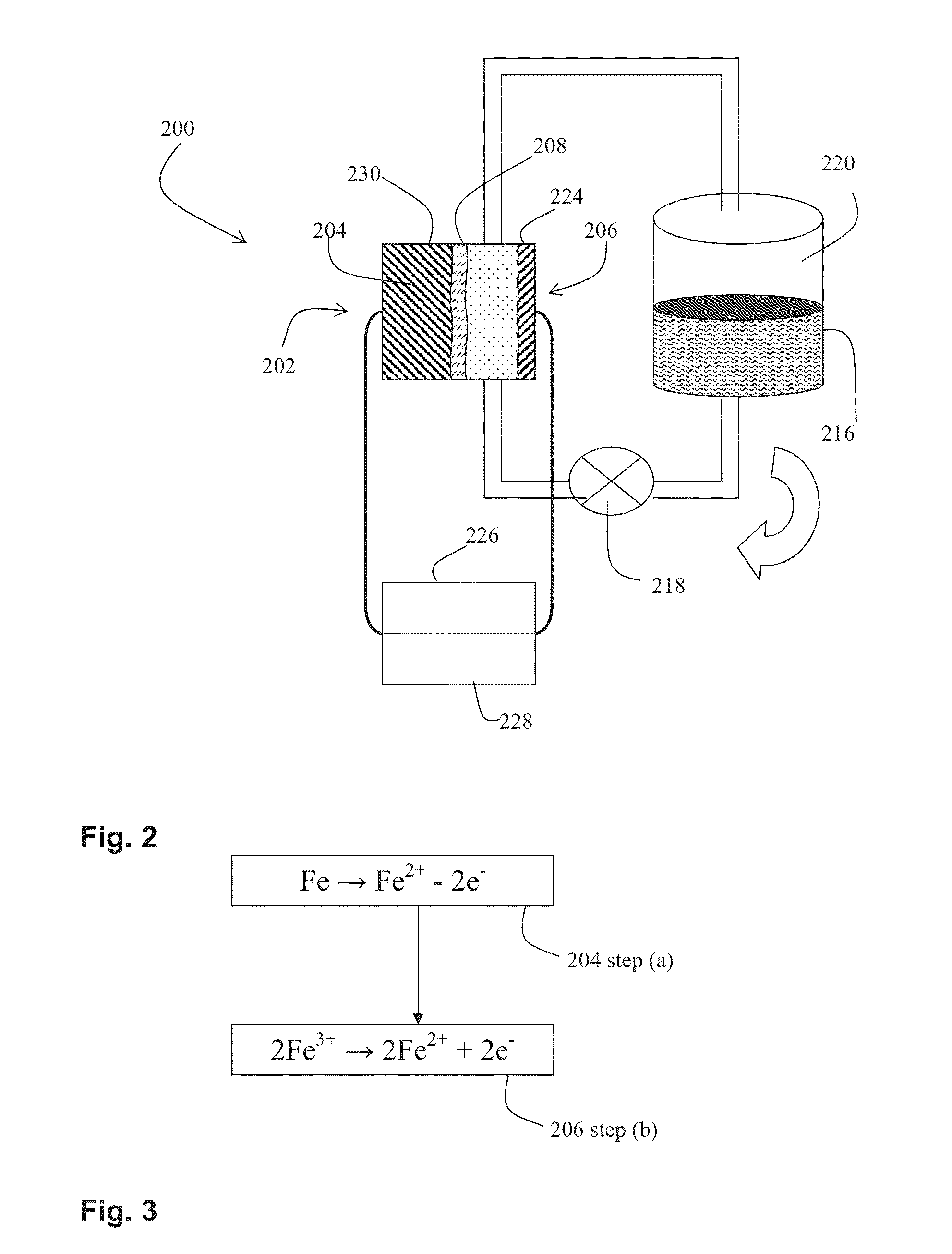
Novel flow battery and usage thereof
InactiveUS20150048777A1Promote redox processPrevent free accessBatteries circuit arrangementsReactant parameters controlCyanideMetal
A voltaic cell comprising an iron in alkali anode where metal iron is oxidized to iron II hydroxide and a ferricyanide in alkali cathode where ferricyanide is reduced to ferrocyanide, and uses thereof.
Owner:EPSILOR ELECTRIC FUEL
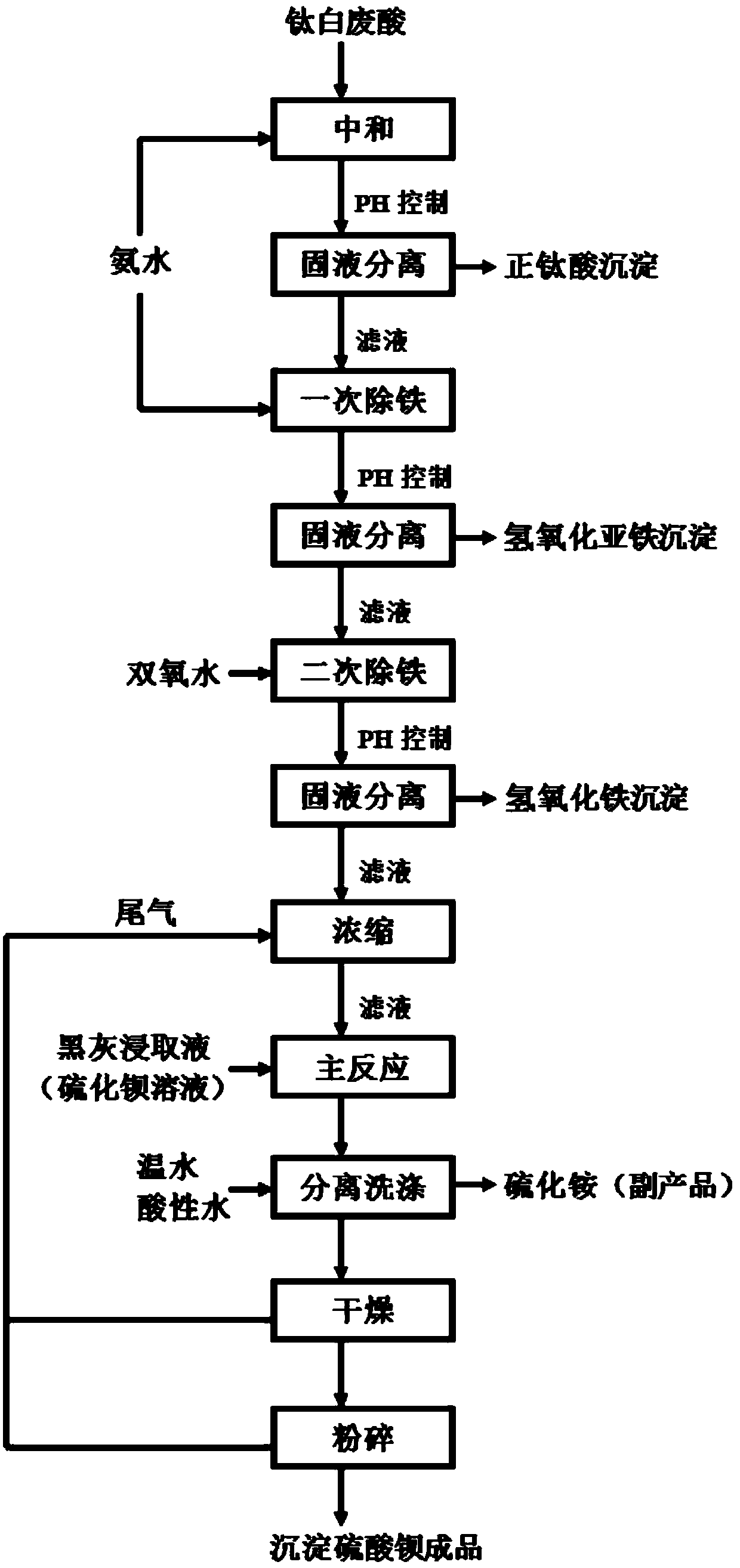
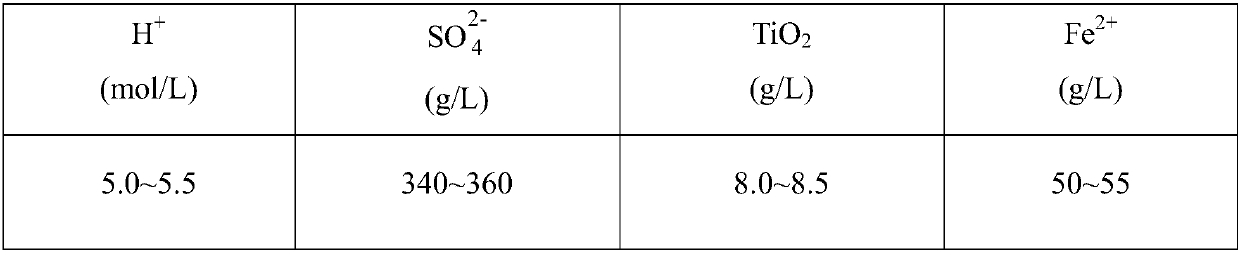
Comprehensive utilization method of titanium white waste acid
Disclosed is a comprehensive utilization method of titanium white waste acid which comprises, (1) neutralizing free sulfuric acid in the waste acid with calcium carbonate, filtering so as to obtain plaster stone dust cake and ferrous sulfate filter liquor, (2) baking the obtained dust cake with rotary kiln or ebullition fluidized-bed plants, obtaining calcium sulphate product, (3) neutralizing the obtained ferrous sulfate solution with caustic soda to obtain ferrous hydroxide, letting in air for oxidizing under normal temperature so as to produce iron red seed crystal, (4) at the presence of seed crystal, heating with steam, carrying out two step oxidization with air, charging ferrous sulfate and caustic soda in a balanced manner, terminating reaction by selecting various time so as to obtain iron-oxide red slurry with different colors, when the reaction ends, subjecting the iron-oxide red slurry to white washing, sieving, filtering, drying and disintegrating, thus obtaining the iron-oxide red product.
Owner:HUBEI WANRUN NEW ENERGY TECH DEV
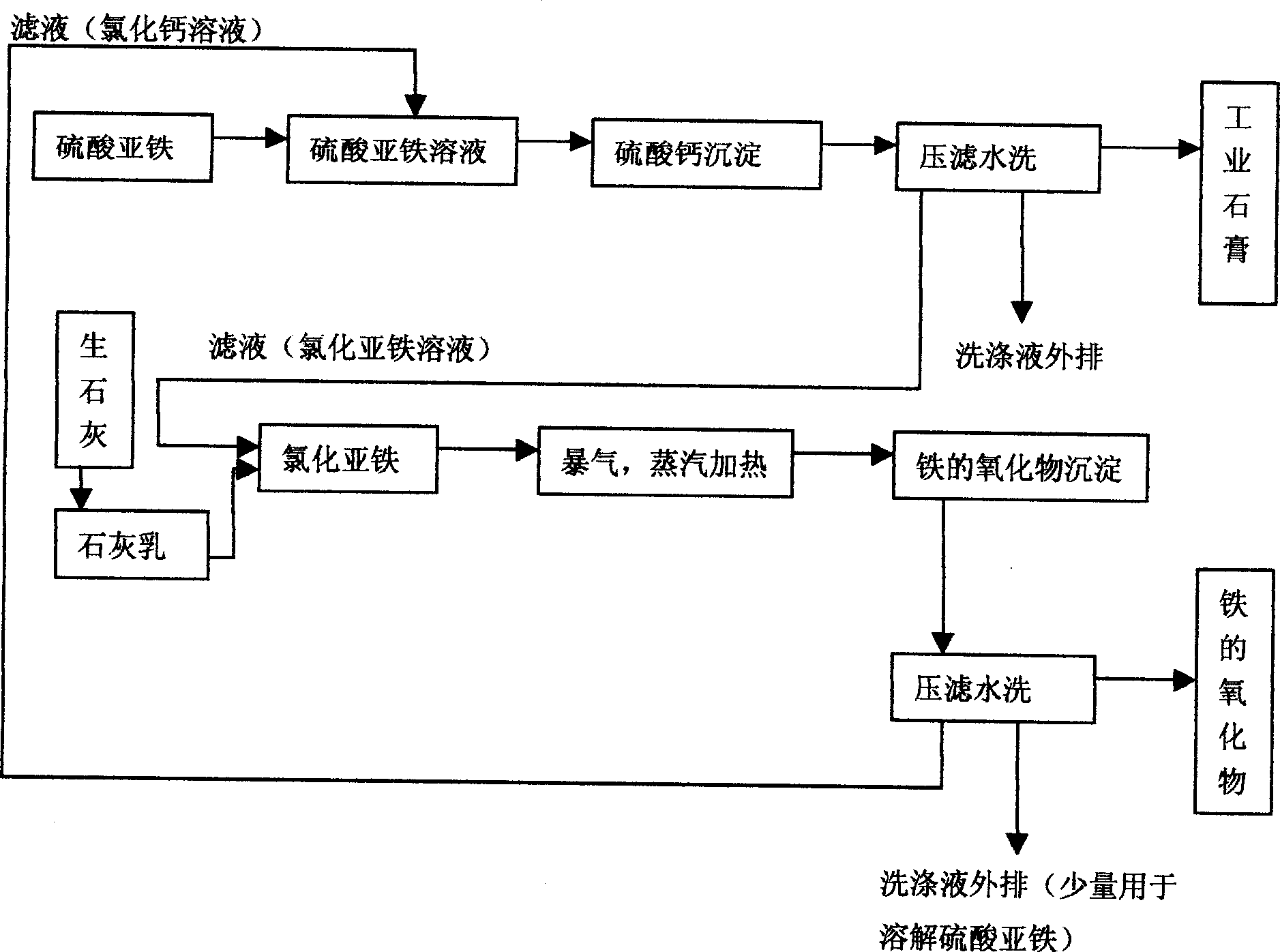
Comprehensively utilizing new process for ferrous sulfate
The technological process of utilizing ferrous sulfate comprehensively in preparing industrial gypsum and iron with lime as main material has the key point of bridging effect of calcium chloride. The technological process includes the steps of: dissolving ferrous sulfate in water, adding calcium chloride solution to produce calcium sulfate precipitate and ferrous chloride solution, filtering to separate calcium sulfate precipitate, adding lime milk into the filtrate and heating via stirring to produce ferric hydroxide precipitate, filtering, stoving the filter slag to obtain ferric oxide, and returning the filtrate. The obtained ferric oxide may be used in smelting iron, preparing iron oxide red, etc. The present invention has relatively low cost of utilizing ferrous sulfate.
Owner:刘应兵
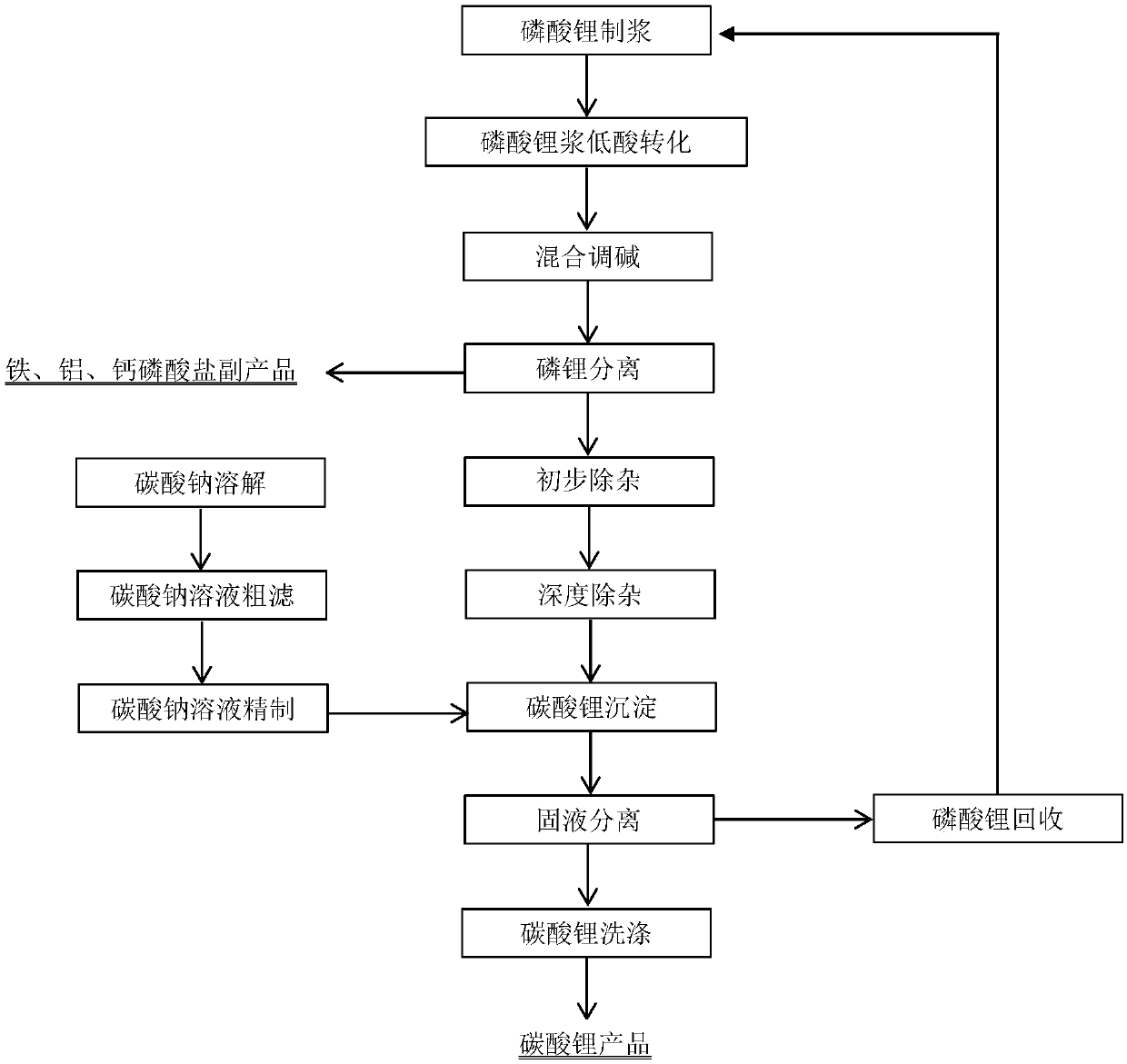
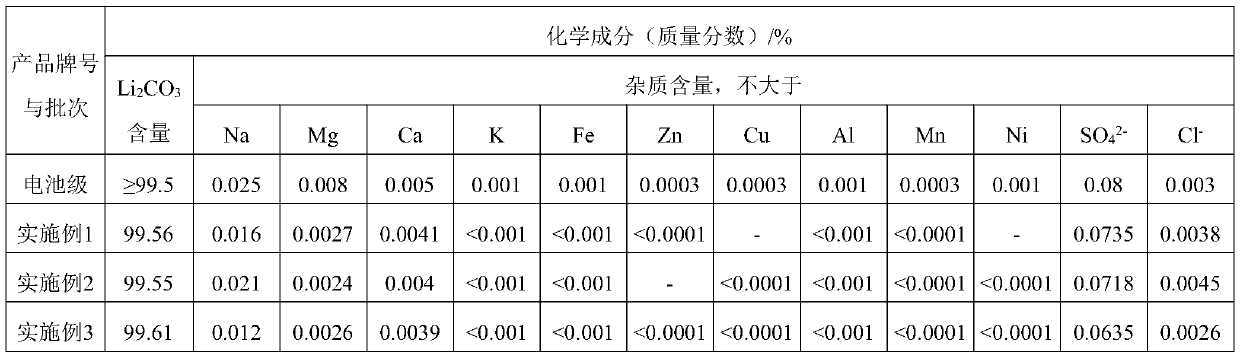
Method for preparing lithium carbonate for batteries by using low-grade lithium phosphate acidity conversion method
ActiveCN108675323AGood removal effectHigh purityLithium carbonates/bicarbonatesFerric hydroxideAluminum Ion
The invention provides a method for preparing lithium carbonate for batteries by using a low-grade lithium phosphate acidity conversion method. The method comprises the following steps: taking solublesalts of iron, ferrous, aluminum and calcium as converters, enabling the converters to sufficiently react with lithium phosphate under a low-acidity condition, and primarily separating lithium ions from phosphate radicals in the lithium phosphate; adjusting the pH value of a composite decomposition reaction product, and precipitating ferric phosphate, ferrous phosphate, aluminum phosphate or calcium hydrophosphate completely; filtering out the precipitate, putting an alkali substance into the filtrate to adjust the pH value of the filtrate, precipitating ferric ions, ferrous ions, aluminum ions or calcium ions in the filtrate to obtain ferric hydroxide, ferrous hydroxide, aluminum hydroxide or calcium hydroxide, and removing the components to obtain a primary purification liquid; performing ion exchange treatment on the primary purification liquid to obtain a deep purification liquid; enabling the deep purification liquid to react with a sodium carbonate solution at 85-100 DEG C, washing, and drying, thereby obtaining battery-grade lithium carbonate.
Owner:GANZHOU NONFERROUS METALLURGICAL RES INST
Resource utilization method for titanium white by-product ferrous sulphate
InactiveCN102351231ASolve pollutionSolve the technical problem that the titanium dioxide by-product ferrous sulfate cannot be processed in large quantities at low costIron oxides/hydroxidesCalcium/strontium/barium sulfatesResource utilizationDihydrate Calcium Sulfate
The invention discloses a resource utilization method for titanium white by-product ferrous sulphate, which solves the technical problem of incomplete utilization of titanium white by-product ferrous sulphate with low cost in the prior art. The method of the invention comprises the following steps: a, purifying and processing titanium white by-product ferrous sulphate; b, introducing ammonia gas in a ferrous sulphate solution obtained by purifying and processing, fully reacting and filtering to obtain ferrous hydroxide deposition and an ammonium sulfate solution; c, adding calcium hydrate in the ammonium sulfate solution and fully reacting to obtain dihydrate calcium sulphate deposition and ammonia gas, recovering ammonia gas as a raw material of a step b, drying the dihydrate calcium sulfate deposition and processing to obtain the product gypsum. According to the invention, sulfur and iron resources in titanium white by-product ferrous sulphate are fully used for producing industrial grade gypsum and iron ore concentrate, the problem of titanium white by-product on environmental pollution is solved; The ammonia gas can be circularly used without consumption based on theory, the loss amount needs to be added in practical production, and the production cost is low.
Owner:攀枝花市尚亿科技有限责任公司
Method for producing high purity battery level iron oxalate from pickle liquor
The invention relates to a method for producing high-purity battery grade iron oxalate by utilizing pickle liquor, comprising the following steps: the pickle liquor is pre-treated for lowering spent acid content, thus leading the pickle liquor to generate ferrite solution with a certain concentration; then after strict filtering and magnetic separation, the ferrite solution is transported into a reaction kettle, then industry ammonia is added and the solution is stirred for reaction to generate ferrous hydroxide gelatin; the soluble oxalic acid solution is slowly added in and stirred, and then the mixture is stirred by a conversion reaction kettle and heat preservation is carried out, thus obtaining iron oxalate slurry after reaction; iron oxalate mother solution is separated in the iron oxalate slurry, and the separated iron oxalate is washed, dried and crashed for obtaining high-purity superfine iron oxalate products. The method has the beneficial effects that: the pickle liquor in industrial production is fully utilized; a two-step liquid phase method of oxidation and conversion is adopted for leading the reaction to be more fully, so that the reaction rate is greatly improved, the grain diameter of the finished products iron oxalate is fine and uniform, D50 is less than or equal to 5mum, and the content of the iron oxalate is more than or equal to 95.5 percent; furthermore, the pickle liquor is used as raw material, and cyclic utilization and environmental protection of the industrial waste are utilized, thus changing waste into valuable and greatly lowering the production cost.
Owner:HUBEI WANRUN NEW ENERGY TECH DEV
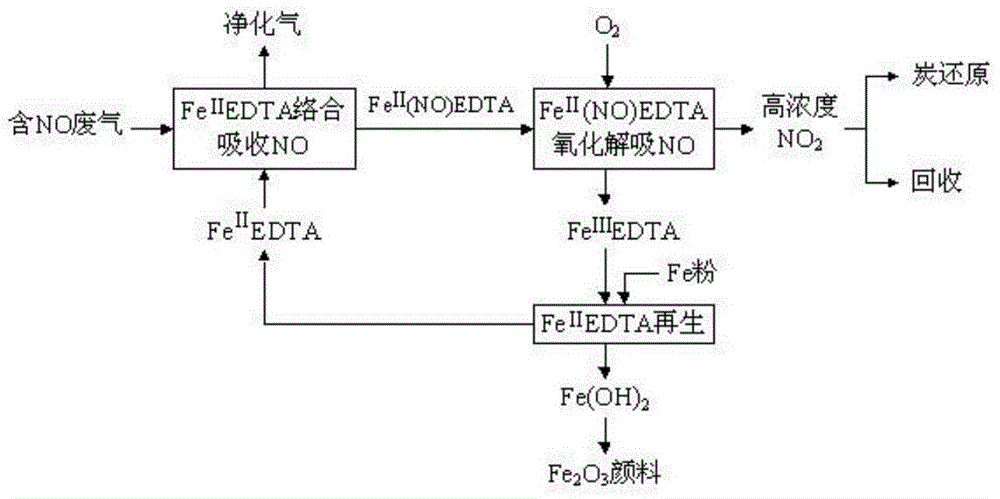
Method for boiler flue gas denitration
ActiveCN104084024AImprove efficiencySimple recycling processDispersed particle separationHigh concentrationDesorption
The invention discloses a method for boiler flue gas denitration, which belongs to the field of environment protection. The method comprises the following steps: complexing and absorbing Fe<II>EDTA (Ethylene Diamine Tetraacetic Acid), performing oxidation and desorption, and reducing and regenerating scrap iron, wherein the step of oxidation and desorption comprises the following substeps: oxidizing Fe<II> (NO)-EDTA into Fe<III>EDTA, which is incapable of absorbing NO, by using air or oxygen to release NO in the complexing and absorption product, namely FeI<II>(NO)EDTA and obtain high-concentration NOx which takes NO2 as a main component, conveying NOx into a boiler hearth, reducing NOx into nitrogen in the process that fuel is combusted in the boiler hearth, and further reducing Fe<III>EDTA obtained in the oxidation and desorption process into Fe<II>EDTA, which is used for circularly absorbing NO, by using the scrap iron, wherein an obtained byproduct, namely ferrous hydroxide, is applied to production of iron oxide red pigments. The method is high in denitration absorption efficiency, high in efficiency, low in transportation cost, convenient to maintain and particularly applicable to boiler flue gas denitration, and the NO desorption and absorbent regeneration process is simple.
Owner:HUNAN PINGAN ENVIRONMENT PROTECTION +1
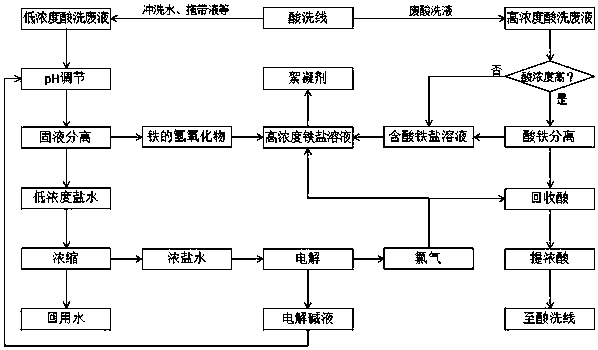
Green recycling method for steel pickling waste liquor
ActiveCN108455680ASolve the problem that the iron content is low and it is not suitable for the preparation of flocculantsIncrease profitElectrolysis componentsIron oxides/hydroxidesHigh concentrationSaline water
The invention discloses a green recycling method for steel pickling waste liquor. The green recycling method is characterized by specially comprising the following steps: neutralizing low-concentration pickling waste liquor with inorganic base, thereby obtaining ferric hydroxide or ferrous hydroxide precipitates and low-concentration saline water; performing acid and iron separation on high-concentration pickling waste liquor, thereby obtaining recycled acid and a ferric acid containing solution; dehydrating ferric hydroxide or ferrous hydroxide precipitates, and then, adding the ferric acid containing solution to obtain high-concentration iron salt solution; concentrating the low-concentration saline water to obtain concentrated saline water and recyclable water; electrolyzing concentrated saline water to prepare chlorine gas and an inorganic base solution; circulating the inorganic base solution for neutralizing pickling waste liquor; leading chlorine gas into the recycling acid to improve concentration of hydrochloric acid, and recycling concentrated recycled acid to a pickling line; and leading chlorine gas into a high-concentration iron salt solution to prepare a flocculatingagent. The green recycling method has the advantages that: a utilization rate of the pickling waste liquor is expected to be greatly increased, emission of three wastes of gas, liquid and solid is avoided throughout the process, and the method is economical, green and environmentally friendly.
Owner:江苏宝钢精密钢丝有限公司 +1
Method for preparing black iron oxide wrapped with silicon dioxide
InactiveCN103333523AImprove water resistanceImprove dispersion stabilityPigment treatment with macromolecular organic compoundsFlocculationWeather resistance
The invention discloses a method for preparing black iron oxide wrapped with silicone dioxide. The method comprises the following steps of firstly adding water and dissolving ferrous sulfate, then adding sodium hydroxide solution to neutralize all ferrous ions, obtaining ferrous hydroxide gel, after oxidizing reaction by aerating air, washing, drying, and smashing so as to obtain the black iron oxide; then taking ethyl orthosilicate as the raw material, forming a layer of continuous compact silicone dioxide film on the surface of the black iron oxide through a sol-gel method, and obtaining the black iron oxide wrapped with the silicone dioxide. The black iron oxide prepared by the method has the characteristics of good acid-base resistance property, weather resistance, compatibility, dispersibility, flocculation resistance, strong tinting strength, and the like, thus being widely used in various paintings and having prosperous market prospect.
Owner:ANHUI TOPTECHSCR ENVIRONMENTAL SCI & TECH
Preparation method of magnetic ferric oxide/bagasse active carbon
ActiveCN104888705ASimple processReduce manufacturing costOther chemical processesAlkali metal oxides/hydroxidesFerric hydroxideSorbent
The invention discloses a preparation method of magnetic ferric oxide / bagasse active carbon. The preparation method comprises the following steps: adding 10-60g of bagasse into 1000mL of 0.05-0.5mol / L ferrous sulfate, stirring at constant speed, carrying out ultrasonic oscillation for 30-60 minutes, and standing for 24-48 hours; slowly adding an ammonia water solution with the volume ratio being 1%-10% by using a full-automatic titrator to adjust the pH value to 8.0-8.5, heating to 85 DEG C by using a microwave oven, filtering, washing with ultrapure water, enabling the pH value of a washing liquid to 7.0, putting a filter cake in a beaker containing 200mL of analytical absolute alcohol, putting the beaker in an ultrasonic instrument for carrying out ultrasonic oscillation for 30 minutes and filtering; drying for 16-24 hours at the temperature of 105-110 DEG C to obtain bagasse / ferric hydroxide and ferrous hydroxide mixture; and carrying out carbonization at the temperature of 450-550 DEG C, cooling, grinding, and sieving by a 100-mesh sieve to obtain a magnetic ferric oxide / bagasse active carbon compound absorbent. The preparation method disclosed by the invention is simple in process and low in cost; and a prepared product can be widely applied in the procedure for deeply treating arsenic-containing waste water of industrial and mining enterprises.
Owner:GUILIN UNIVERSITY OF TECHNOLOGY
Method for preparing iron oxide red by utilizing iron-containing wastes and iron oxide red pigment
The invention relates to the technical field of chemical industry and provides a method for preparing iron oxide red by utilizing iron-containing wastes. The method comprises the following steps: converting an iron element in the iron-containing wastes into ferric hydroxide, ferrous hydroxide or a mixture of ferric hydroxide and ferrous hydroxide; then carrying out constant-temperature drying for3 to 5h at 120 to 180 DEG C in the presence of oxygen gas, so as to obtain dry powder; storing the dry powder for 40 to 180min under a sealed condition at 400 to 800 DEG C, so as to obtain gamma-Fe2O3. The method provided by the invention can be used for recycling the iron-containing wastes and resources are avoided; the yield of iron oxide red is high and the economic benefits are relatively high. The invention further provides an iron oxide red pigment; the iron oxide red pigment is obtained through gamma-Fe2O3 in a standardized manner; iron oxide red pigment has relatively excellent economic benefits.
Owner:CHANGZHOU SANLE ANTICORROSIVE MATERIAL CO LTD
Method for recycling copper and nickel in electroplating sludge
InactiveCN108611491ALess investmentMature technologyProcess efficiency improvementFerric hydroxideSludge
The invention provides a method for recycling copper and nickel in electroplating sludge. A separation method comprises the steps of 1, separation of copper, wherein a sodium carbonate solution is adopted for adjusting the pH of leachate called mother liquor to 5-5.5, and filtered sediments are mainly basic cupric carbonate and copper hydroxide; 2, separation of iron and nickel, wherein a sodium carbonate solution is utilized for adjusting the pH of the mother liquor to 8-9, sediments are nickel carbonate, ferric hydroxide and ferrous hydroxide, sulfuric acid and hydrogen peroxide are added tofully dissolve the sediments, afterwards, the pH is adjusted to 5 through alkaline liquor, and a sediment, namely ferric hydroxide, is filtered out; 3, purification and separation of a nickel product, wherein the filtered mother liquor is taken, the pH is adjusted by sulfuric acid to 2, hydrogen sulfide is introduced, copper in mixed liquor is turned into copper sulphide and separated out, at this moment, the mother liquor is nickel sulfate mother liquor, and nickel sulfate heptahydrate can be produced through a concentration and crystallization method. The method has the advantages that theinvestment is small, the technology is mature, the adaptability is high, and the automated degree is high.
Owner:TIANJIN RUISAIKE NEW MATERIAL TECH CO LTD
Method for preparing precipitated barium sulfate by utilizing titanium white waste acid
ActiveCN107720801AReduce cost pressureReduce pressure on environmental protectionAmmonium sulfides/polysulfidesCalcium/strontium/barium sulfatesFerric hydroxideSodium sulfate
The invention discloses a method for preparing precipitated barium sulfate by utilizing titanium white waste acid, belonging to the technical field of comprehensive utilization of titanium white wasteacid and preparation of barium sulfate. The method comprises the following steps of adding ammonium hydroxide to neutralize with titanium white waste acid firstly, then generating ammonium sulfate, and controlling a systemic pH to separate out photodegredation sediment; then adding the ammonium hydroxide continuously, and controlling the pH to separate out ferrous hydroxide sediment; then addinghydrogen peroxide in proportion, oxidating residual Fe2<+> into Fe3<+>, and adjusting the pH to separate out ferric hydroxide sediment; condensing an ammonium sulfate solution, then replacing a mirabilite solution in a traditional precipitated barium sulfate production process, enabling the ammonium sulfate solution to generate replacement reaction with a barium sulfide solution, and washing, drying and smashing to prepare a precipitated barium sulfate product. According to the method, after neutralizing the titanium white waste acid with the ammonium hydroxide and performing purification andimpurity removal, a traditional sodium sulfate solution is replaced for producing the precipitated barium sulfate, meanwhile, recycled photodegredation sediment can be further used for titanium dioxide production, not only are resources saved, but also waste can be recycled, and the waste acid treatment costs of titanium dioxide plants and environment protection pressure are reduced.
Owner:ANHUI JXTB GRP
Method for preparing iron oxide yellow
The invention relates to a method for preparing iron oxide yellow, which comprises the following steps: step 1: using sulfuric acid to react with iron in a reaction tank to prepare an FeSO4 solution; step 2: under the condition of stirring, adding the FeSO4 solution prepared in the step 1 as well as an NaO solution and a deflocculant solution into a sedimentation tank simultaneously to prepare a ferrous hydroxide sizing agent; step 3: under the condition of stirring, leading air in the sedimentation tank to oxidize the ferrous hydroxide sizing agent prepared in the step 2 to obtain a ferric hydroxide sizing agent; and step 4: washing, filtering, drying and grinding the ferric hydroxide sizing agent prepared in the step 3 to obtain the iron oxide yellow product. According to the invention, the method is simpler, is more environment-friendly, and the spherical iron oxide yellow can be prepared.
Owner:张学政
Preparation method for iron sodium ethylene diamine tetraacetate
ActiveCN105294469APrecipitation particles are largeHigh reactivityOrganic compound preparationAmino-carboxyl compound preparationEthylenediamineAcetic acid
The invention discloses a preparation method for iron sodium ethylene diamine tetraacetate. The preparation method comprises the following steps: (1) weighing a predetermined amount of ferrous sulfate heptahydrate, adding ferrous sulfate heptahydrate into deionized water, weighing a predetermined amount of bicarbonate, carbonate or a mixture of bicarbonate and carbonate, adding the weighed bicarbonate, carbonate or mixture into a ferrous sulphate solution, carrying out a reaction so as to obtain large-grain ferrous hydroxide precipitate and introducing air to completely oxidize ferrous hydroxide into reddish brown iron hydroxide powder for subsequent usage; (2) weighing a predetermined amount of ethylene diamine tetraacetic acid and sodium hydroxide and adding ethylene diamine tetraacetic acid and sodium hydroxide into deionized water; and (3) addin gthe iron hydroxide powder into the solution obtained in the step (2) under stirring according to a predetermined mol ratio, adjusting a pH value to 4.5 to 6.0, heating the solution to 60 to 80 DEG C, carrying out stirring reaction for 30 to 50 min and subjecting the solution to filtering and crystallization so as to obtain finished iron sodium ethylene diamine tetraacetate. The method provided by the invention prepares iron hydroxide through on-site oxidation of ferrous hydroxide, and the prepared iron hydroxide has high reaction activity, needs short time for subsequent chelation reaction and enables product yield to be high and production efficiency to be greatly improved.
Owner:上海永通生态工程股份有限公司
Method for preparing raw material of desulfurizing agent by using waste chlorination molten salt generated in production of TiCl4
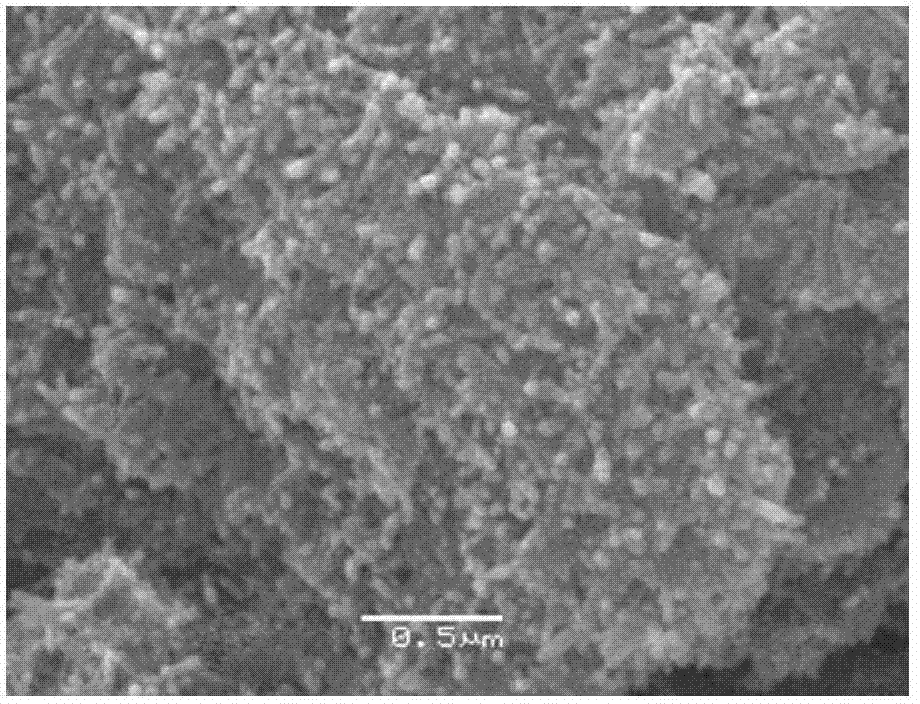
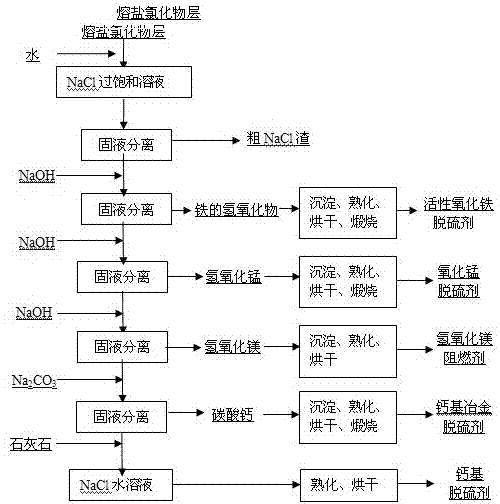
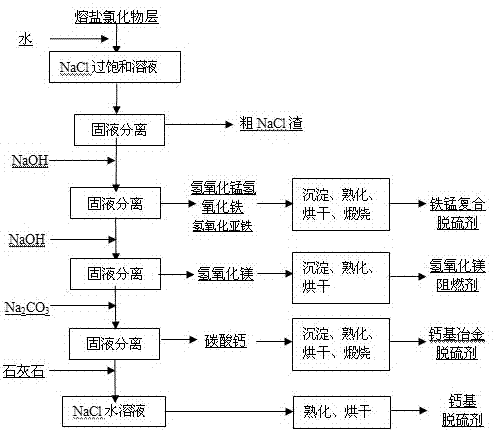
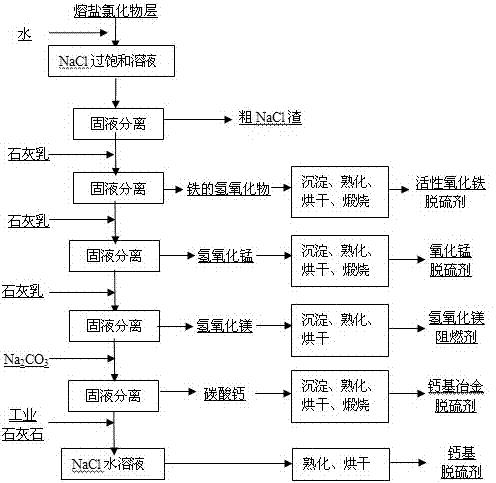
InactiveCN103566719AAchieve regenerationRealize comprehensive utilizationSolid waste disposalDispersed particle separationElectrolysisManganese
The invention discloses a method for preparing a raw material of a desulfurizing agent by using waste chlorination molten salt generated in production of TiCl4, and relates to a method for treating waste molten salt and wastewater containing Cl<->. The method comprises the following steps: performing water solution treatment on a crushed waste molten salt chloride layer, filtering out dregs, subsequently performing treatment of iron removal, manganese removal, magnesium removal and calcium removal on the obtained water solution of the chloride step by step, so as to respectively obtain solutions of ferric hydroxide, ferrous hydroxide, manganese hydroxide, magnesium hydroxide, CaCO3 and NaCl, treating ferric hydroxide, ferrous hydroxide, manganese hydroxide, magnesium hydroxide and CaCO3, subsequently preparing a series of desulfurizing agents, and taking the NaCl aqueous solution as a modifying agent of a calcium-based desulfurizing agent. Due to the adoption of the method, the problem of environment pollution of waste molten salt and wastewater containing Cl<-> in preparation of TiCl4 by using a molten salt electrolysis method, which plagues people for a long time, is solved, the waste molten salt can be prepared into iron-based, magnesium-based, ferro-manganese-based and calcium-based desulfurizing agents for treating a great deal of industrial wastewater containing NaCl, and recycling utilization of the waste molten salt and the industrial wastewater containing NaCl is achieved.
Owner:重庆阁林环保科技有限公司
Processing method of radioactive waste liquid containing uranium
InactiveCN102930914AReduce concentrationReduce pollutionRadioactive decontaminationIonIron(II) hydroxide
The invention discloses a processing method of radioactive waste liquid containing uranium. The processing method includes: an iron ion solution or a ferrous ion solution is added in the waste liquid containing radioactive substance uranium, pH value is controlled by an alkaline solution, ferric hydroxide or ferrous hydroxide colloid is generated to enable the radioactive element uranium and the colloid to form coprecipitation, astringent persimmon tannin is added to enable the colloid to form large-particle precipitation, and filtration is performed. The processing method of the radioactive waste liquid containing the uranium has strong purifying capacity for the waste liquid containing the radioactive substance uranium, and enables content of the radioactive element uranium in the waste liquid to be zero after processing.
Owner:GUILIN UNIV OF ELECTRONIC TECH
Magnetcisuspension polycoagulant, preparation method and application
InactiveCN1796301AExcellent flocculation and turbidity reductionGood degreasing effectWater/sewage treatment by flocculation/precipitationRare earthAluminum silicate
This invention discloses the preparation and application of a magnetic suspension flocculant, which is composed of 30-60 portions of 5-15% polyacrylic acid solution (molecular weight 600,000,000-800,000,000), 20-40 portions of 20-40% polyaluminum silicate solution, 2-10 portions of ferrous hydroxide, 2-10 portions of diatomite and 10-30 portions of water. The flocculant exhibits good performance in flocculation as well as in oil removing. The flocculant of this invention can flocculate the magnetic and non-magnetic particles in the water into large grains, and can absorb the oil from the water, which can then be removed with rare earth magnetic discs. The combination of rare earth magnetic discs and the magnetic suspension flocculant has such advantages as a short processing flow, a small floor area, a low cost and a high oil-removing rate, which can be widely used for treating wastewater containing suspended substances and oil.
Owner:北京博泰盛合科技有限公司
Method for controlling production of precipitation-method iron red mixed crystals
InactiveCN103342391ASimple stepsQuick checkFerric oxidesColor/spectral properties measurementsIr microscopeAbsorbance spectra
The invention discloses a method for controlling production of precipitation-method iron red mixed crystals. The method comprises the following steps of: (1), quickly oxidizing ferrous hydroxide precipitate which is added with a dispersing agent by an oxidizing agent to prepare iron red seed crystals; (2), drying the iron red seed crystals, tabletting, scanning and processing to obtain an infrared spectrogram; (3), obtaining an iron red seed crystal characteristic peak position and an impurity characteristic peak position according to an IR (Infrared Radiation) absorption spectrum characteristic peak of oxyhydroxide of the iron, and judging the quality of the iron red seed crystal sample according to the strength of the impurity peak; and (4), carrying out two-step oxidization on the seed crystals according to the infrared analyzed result, carrying out infrared detection on the oxide product iron red to obtain an infrared spectrogram of the iron red, and judging the quality of the iron red according to the strength of the impurity peak.
Owner:南通宝聚颜料有限公司
Method for preparing industrial ferrous sulfate and zinc sulfate by using pyrite cinders
The invention provides a method for preparing industrial ferrous sulfate and zinc sulfate by using pyrite cinders. The method comprises the following steps of: fully reacting the pyrite cinders with excessive sulfuric acid, filtering to obtain a mixed solution I containing sulfuric acid, ferrous sulfuric, cuprous sulfate and zinc sulfate, and a lead-containing filter cake; adding an appropriate amount of ferrous hydroxide into the mixed solution I for neutralizing the excessive sulfuric acid; distilling the solution under a reduced pressure into a saturated ferrous sulfate solution, cooling the saturated ferrous sulfate solution to 25 DEG C until the ferrous sulfate is supersaturated, separating a large quantity of crystals out, and filtering to obtain a ferrous sulfate filter cake and a mixed solution II containing cuprous sulfate pentahydrate, zinc sulphate heptahydrate and a small amount of ferrous sulfates; washing, drying and smashing the ferrous sulfate filter cake to obtain a ferrous sulfate product; heating the mixed solution II to 30-40 DEG C, precipitating zinc sulfate in a form of zinc sulfate heptahydrate crystals, and filtering to obtain zinc sulfate heptahydrate crystals and a mixed solution III containing copper sulfate and ferrous sulfate; and washing, drying and smashing the zinc sulfate heptahydrate crystals to obtain a zinc sulfate crystal product.
Owner:张胜勇
Preparation method of industrial water fluorine removal agent
ActiveCN104085973AGood defluoridation effectHigh fluoride removal efficiencyWater/sewage treatmentEthylenediamineSodium hydroxide
The invention discloses a preparation method of an industrial water fluorine removal agent, and belongs to the technical field of industrial water fluoride removal. The industrial water fluorine removal agent is prepared from the following synthetic raw materials: acrylic acid, de-ionized water, acrylamide, urea, sodium carbonate, ferrous hydroxide, aluminum hydroxide, 10%-30% sodium hydroxide, active carbon, 10% ethylenediamine, nitrilo, ammonium persulfate, sodium hydrogen sulfite and calcium chloride. The preparation method comprises the following steps: preparing the synthetic raw materials, polymerizing and mixing to prepare the industrial water fluorine removal agent. The preparation method has the advantages that the prepared fluorine removal agent is high and stable in fluorine removal efficiency, and has a good fluorine removal effect on low-fluorine water with fluoride content of 20-50mg / L; a medicament preparation process is simple.
Owner:SHOUGANG CORPORATION
Method for comprehensively recovering rare and dispersed noble metal from refining furnace slag
InactiveCN106222431ASimple process equipmentReduce energy consumptionProcess efficiency improvementZinc hydroxideIron(II) oxide
The invention relates to a method for comprehensively recovering rare and dispersed noble metal from refining furnace slag, and belongs to the technical field of comprehensive recovery of rare and dispersed noble metal. Rare and dispersed metal is leached out through a hydrochloric acid-chlorine salt system, tin and antimony in leachate are removed through precipitation by precipitants including ferrous hydroxide, ferrous oxide, zinc hydroxide, zinc oxide and the like, and iron powder or zinc powder is used as a displacer used for precipitating bismuth in liquid obtained after tin and antimony precipitation, so that coarse bismuth is prepared; and lead in hydrochloric acid-chlorine salt leaching cinder is extracted through a conversion-leaching process, and noble metal such as gold and silver is enriched in slag which is returned for smelting. The tin-antimony enrichment ratio reaches 14% or over, and the precipitation rate is 99.9%; the bismuth leaching rate reaches 99.5% or over, and the direct recovery rate reaches 95% or over; and the gold-silver enrichment ratio is three or more times of the original ratio, and the recovery rate reaches 99%. The process and equipment are simple, energy consumption is low, the comprehensive resource recovery rate is high, the environment is protected, and organic connection with production of an existing factory is well achieved.
Owner:BEIJING GENERAL RES INST OF MINING & METALLURGY
Method for producing iron oxide black by using waste hydrochloric acid solution
The invention provides a method for producing iron oxide black by using a waste hydrochloric acid solution. The method comprises the following steps: 1, purifying the waste hydrochloric acid solution: taking the waste hydrochloric acid solution of a steel plant, adjusting the pH value, adding a certain amount of waste sheet iron, carrying out controlled temperature hydrolysis, adding a flocculating agent after the above reaction is stopped, fully stirring, and taking the obtained supernatant to obtain a ferrous chloride solution; 2, adding liquid alkali, controlling the temperature in a range of 20-30DEG C and the pH value in a range of 6.5-7.5, fully reacting, filtering, and collecting the obtained precipitate; 3, reacting iron oxide yellow having a same mole with ferrous hydroxide with a product obtained in step 2 to obtain crude iron oxide black; and 4, purifying. The method uses the waste hydrochloric acid solution of the steel plant and uses recovered iron oxide red and iron oxide yellow to realize wastewater treatment precipitation, so the method realizes waste reuse, energy saving and environmental protection; and a product obtained through the method has strong coloring power, pure color, and increased purity and yield.
Owner:天津市华创盛达科技有限公司
Preparation method of composite reinforced steel shots
InactiveCN107400500AReduce mechanical damageGood porous shapePulp properties modificationOther chemical processesPorosityFiber
The invention relates to a method for preparing composite reinforced steel shots, which belongs to the technical field of metal product preparation. In the present invention, the coconut shell fiber is boiled and then fermented to increase the fiber porosity, crushed and refined after being frozen in liquid nitrogen, and then modified by soaking in dopamine solution to improve the adsorption performance of the fiber. After drying, react at high temperature under the protection of argon to form a carbonized mixture containing silicon carbide and carbon, and then use the carbonized mixture with a porous structure to absorb and fill the ferrous hydroxide produced by the precipitation. After filtering and drying , and reduced in a hydrogen atmosphere to obtain a composite reinforced steel shot. The invention makes full use of the silicon carbide and carbonaceous components formed at high temperature as reinforcements, improves the impact resistance and wear resistance of the steel shot, prevents the surface of the steel shot from cracking, and the obtained steel shot has a narrow particle size distribution and a spherical or similar shape. The spherical shape effectively solves the problem of short service life of traditional reinforced steel shot.
Owner:常州兆威不锈钢有限公司
Method for producing high purity battery level iron oxalate from pickle liquor
ActiveCN101462942BHigh puritySmall particle sizeCarboxylic acid salt preparationOXALIC ACID DIHYDRATEFerrous salts
The invention relates to a method for producing high-purity battery grade iron oxalate by utilizing pickle liquor, comprising the following steps: the pickle liquor is pre-treated for lowering spent acid content, thus leading the pickle liquor to generate ferrite solution with a certain concentration; then after strict filtering and magnetic separation, the ferrite solution is transported into a reaction kettle, then industry ammonia is added and the solution is stirred for reaction to generate ferrous hydroxide gelatin; the soluble oxalic acid solution is slowly added in and stirred, and then the mixture is stirred by a conversion reaction kettle and heat preservation is carried out, thus obtaining iron oxalate slurry after reaction; iron oxalate mother solution is separated in the iron oxalate slurry, and the separated iron oxalate is washed, dried and crashed for obtaining high-purity superfine iron oxalate products. The method has the beneficial effects that: the pickle liquor in industrial production is fully utilized; a two-step liquid phase method of oxidation and conversion is adopted for leading the reaction to be more fully, so that the reaction rate is greatly improved,the grain diameter of the finished products iron oxalate is fine and uniform, D50 is less than or equal to 5mum, and the content of the iron oxalate is more than or equal to 95.5 percent; furthermore, the pickle liquor is used as raw material, and cyclic utilization and environmental protection of the industrial waste are utilized, thus changing waste into valuable and greatly lowering the production cost.
Owner:HUBEI WANRUN NEW ENERGY TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com