Method for controlling production of precipitation-method iron red mixed crystals
A technology for controlling precipitation and iron red, applied in iron oxide, iron oxide/iron hydroxide, color/spectral characteristic measurement, etc., can solve problems such as yellowish color, loss of iron red pigment performance, and inability to adjust oxidation condition parameters in time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Take 100ml of ferrous hydroxide precipitate with a ferrous iron concentration of 0.1mol / L at 20°C, add 0.05g of dispersant sodium phosphate, blow in air (flow rate 60L / h), react for 1h to obtain iron red seed crystals.
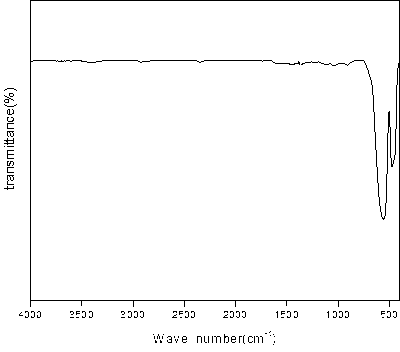
[0024] The iron red seed crystal samples were dried and ground under dark conditions at a temperature of 30°C. Weigh 10 g of dry potassium bromide, add 0.05 g of iron red seed crystal samples, grind, press, and scan to obtain an infrared spectrum of iron red seed crystal samples, and perform atmosphere compensation, baseline correction, and smoothing of the spectrum. By analyzing the infrared spectrum of the iron red seed sample, it is found that the wave number is 1127cm -1 , 945cm -1 The characteristic peak of iron red seed crystal appears at 791cm -1 , 899cm -1 There is no characteristic peak, indicating that there is no iron yellow impurity generated in the iron red seed crystal sample. The seed sample is a pure phase iron red seed crystal, which is of ...
Embodiment 2
[0027] Take 100ml of ferrous hydroxide precipitate with ferrous iron concentration of 0.1mol / L at 20°C, add 0.08g of dispersant stearic acid, blow in air (flow rate 60L / h), react for 1h to obtain iron red seed crystals.
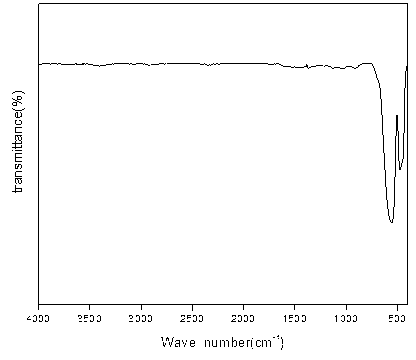
[0028] The iron red seed crystal samples were dried and ground under dark conditions at a temperature of 30°C. Weigh 10 g of dry potassium bromide, add 0.05 g of iron red seed crystal sample, grind, press, and scan to obtain the infrared spectrum of the iron red seed crystal sample, and perform atmosphere compensation, baseline correction, and smoothing of the spectrum. By analyzing the infrared spectrum of the iron red seed sample, it is found that the wave number is 1127cm -1 , 945cm -1 The characteristic peak of iron red seed crystal appears at the place, and the wave number is 791cm -1 , 899cm -1 The characteristic peak of yellow iron mixed crystal appears at the place, but the intensity of the mixed crystal peak is smaller than the characteristic peak of iro...
Embodiment 3
[0031] Take 100ml of ferrous hydroxide precipitate with a ferrous iron concentration of 0.1mol / L at 20°C, add 0.05g of dispersant sodium phosphate, blow in air (flow rate 60L / h), react for 1h to obtain iron red seed crystals.
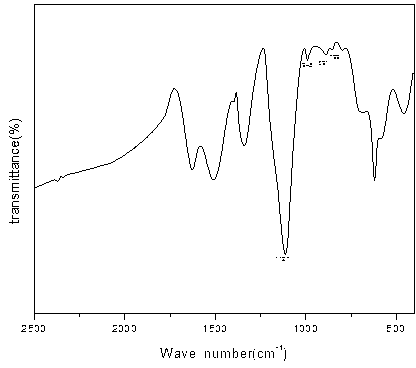
[0032] The iron red seed crystal samples were dried and ground under dark conditions at a temperature of 30°C. Weigh 10 g of dry potassium bromide, add 0.05 g of iron red seed crystal sample, grind, press, and scan to obtain the infrared spectrum of the iron red seed crystal sample, and perform atmosphere compensation, baseline correction, and smoothing of the spectrum. By analyzing the infrared spectrum of the iron red seed sample, it is found that the wave number is 1127cm -1 , 945cm -1 The characteristic peak of iron red seed crystal appears at the place, and the wave number is 791cm -1 , 899cm -1 The characteristic peak of yellow iron mixed crystal appears at the place, and the peak intensity of the mixed crystal peak is larger than the characteristic p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com