Method for preparing lithium carbonate for batteries by using low-grade lithium phosphate acidity conversion method
A technology of lithium phosphate and lithium carbonate, applied in lithium carbonate;/acid carbonate and other directions, can solve the problem of low purity of lithium carbonate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
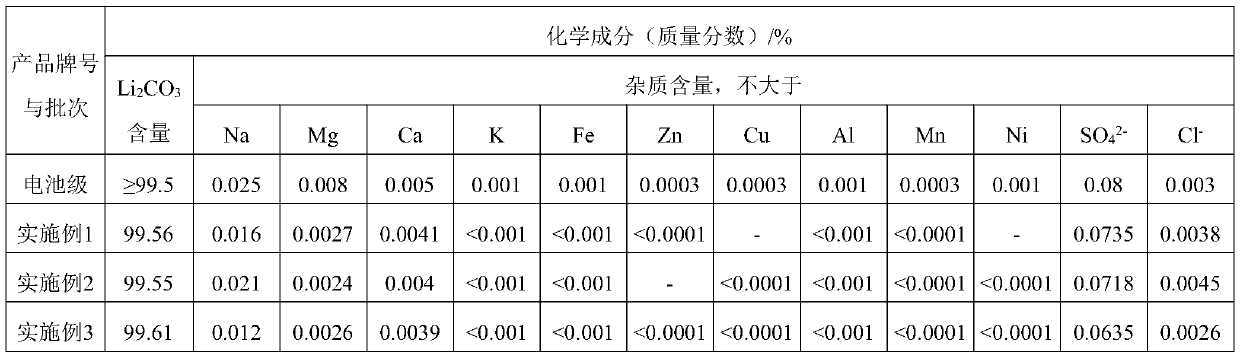
Embodiment 1
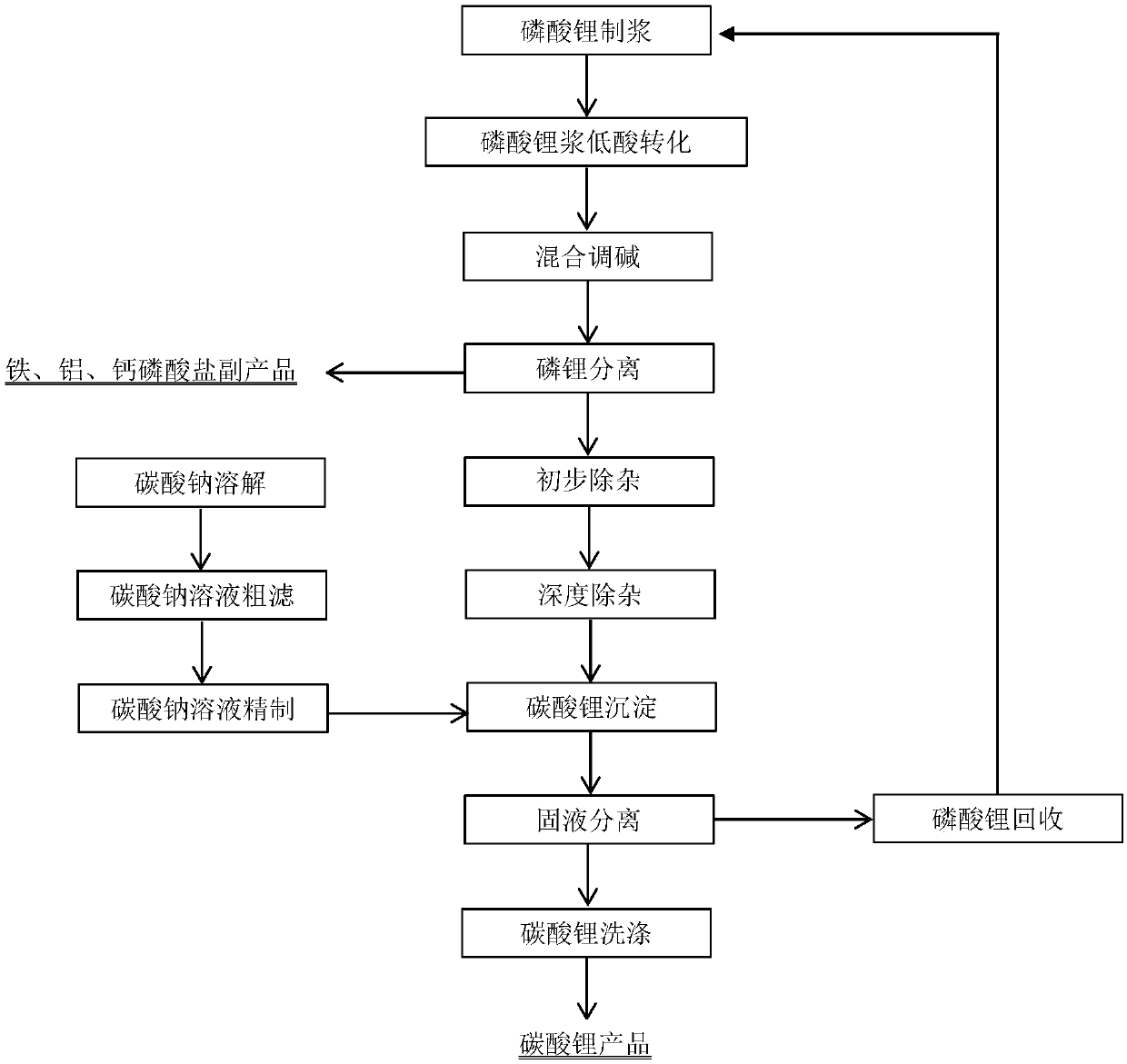
[0109] Weigh 100g of lithium phosphate and pour it into a 2L beaker, add 600ml of water to stir the slurry, slowly add hydrochloric acid and ferric chloride solution, wherein the molar ratio of phosphate, iron ion and hydrochloric acid is 1:1.0:0.9, stir for 1h, the liquid The alkali adjusts the pH value to 2.8, and continues the reaction at 80°C for 0.5h to complete the crystallization of iron phosphate. Solid-liquid separation to obtain the filtrate, that is, crude lithium chloride solution, the solid is iron phosphate, and the iron phosphate is washed with water and then filtered again to obtain the by-product of iron phosphate. The pH value of the crude lithium chloride solution was adjusted to 3.6 with sodium hydroxide solution, and the iron ions were removed by filtration, and the filtrate was a preliminary purification solution. The preliminary purification solution is obtained by ion exchange to obtain a deeply purified lithium solution. After the sodium carbonate is d...
Embodiment 2
[0111] Weigh 100g of lithium phosphate and pour it into a 2L beaker, add 550ml of water to stir and adjust the slurry, slowly add sulfuric acid and ferrous sulfate solution, wherein the molar ratio of phosphate, ferrous ion and sulfuric acid is 1:0.95:0.5, stir for 1h, The liquid caustic soda adjusts the pH value to 6.2, and continues the reaction at 60°C for 0.5h to complete the precipitation of ferrous phosphate. Solid-liquid separation to obtain the filtrate, that is, crude lithium sulfate solution, the solid is ferrous phosphate, and the ferrous phosphate is washed with water and filtered again to obtain the by-product of ferrous phosphate. The pH value of the crude lithium sulfate solution was adjusted to 9.8 with sodium hydroxide solution, and the ferrous ions were removed by filtration, and the filtrate was a preliminary purification solution. The preliminary purification solution is obtained by ion exchange to obtain a deeply purified lithium solution. After the sodium...
Embodiment 3
[0113] Weigh 100g of lithium phosphate and pour it into a 2L beaker, add 500ml of water to stir the slurry, slowly add hydrochloric acid and calcium chloride solution, wherein the molar ratio of phosphate, calcium ion and hydrochloric acid is 1:1.2:0.8, stir for 0.5h, The liquid caustic soda adjusts the pH value to 5.5, and continues the reaction at room temperature for 0.5 h to complete the crystallization of calcium hydrogen phosphate. Solid-liquid separation to obtain filtrate, that is, crude lithium chloride solution, the solid is calcium hydrogen phosphate, and the calcium hydrogen phosphate is washed with water and filtered again to obtain calcium hydrogen phosphate by-product. The pH value of the crude lithium chloride solution was adjusted to 12 with sodium hydroxide solution, and calcium ions were removed by filtration, and the filtrate was a preliminary purification solution. The preliminary purification solution is obtained by ion exchange to obtain a deeply purifie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com