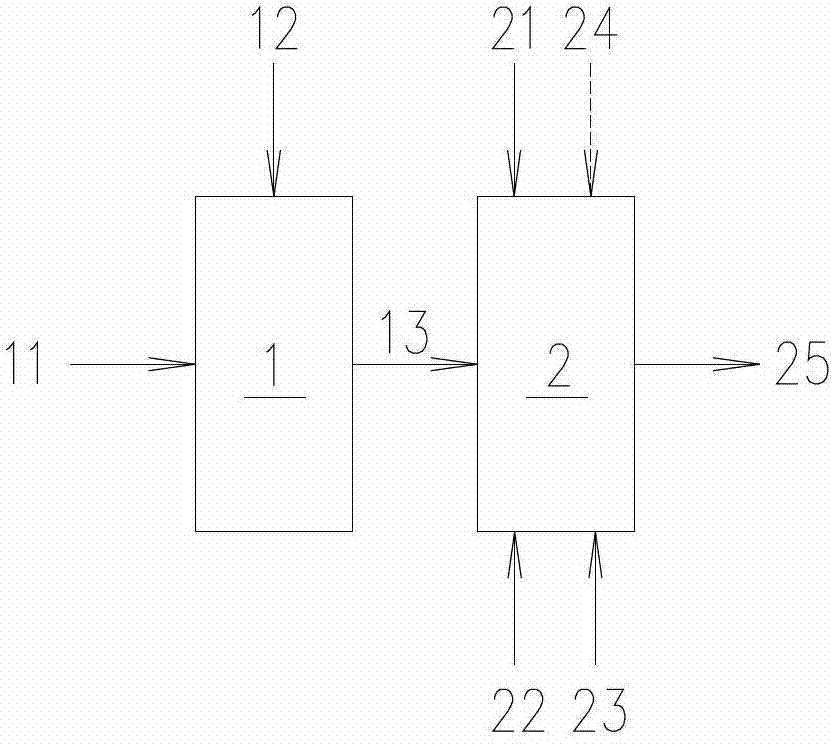Method for preparing iron oxide yellow
A technology of iron oxide yellow and solution, applied in iron oxide, iron oxide/iron hydroxide and other directions, can solve problems such as trouble and water pollution, achieve environmental friendliness, simplify production methods, and avoid the impact of iron oxide yellow product quality. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The method for preparing iron oxide yellow of the present invention will be further described below in conjunction with the accompanying drawings.
[0031] figure 1 It is a schematic process flow diagram of the method for preparing iron oxide yellow according to the present invention. First add iron 11 and sulfuric acid 12 into reaction tank 1 to prepare FeSO 4 Solution 13. Then, adjust the prepared FeSO 4 The concentration of the solution 13 reaches the required concentration and is passed into the sedimentation tank 2. At the same time, the NaOH solution 21, the deflocculant 22 and the additive 23 are added to the sedimentation tank 2, and nitrogen gas is passed through to generate hydrogen under stirring conditions. Ferrous oxide slurry. After the reaction in the sedimentation tank 2 finishes, feed air 24 thereinto, as figure 1 shown by the dotted line in . Under stirring conditions, the ferrous hydroxide slurry was oxidized to obtain ferric hydroxide slurry 25...
Embodiment 1
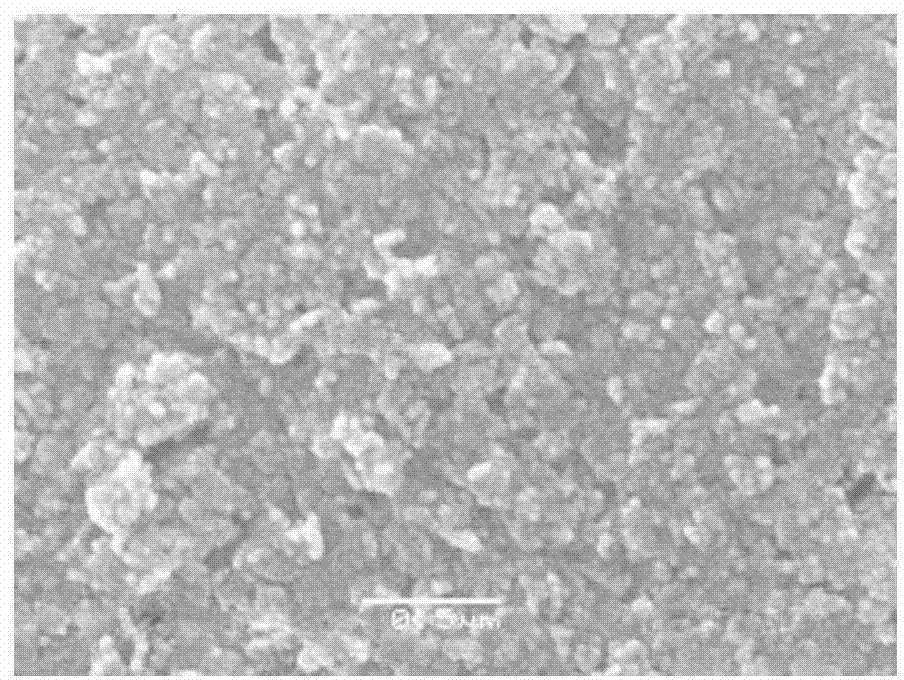
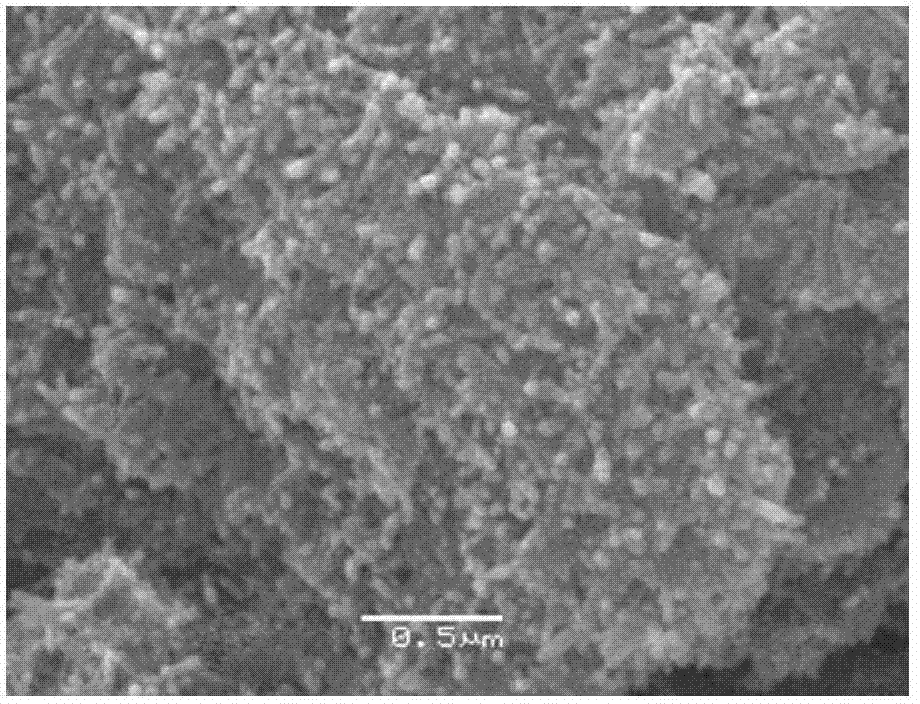
[0033] Prepare FeSO with 0.1mol / L dilute sulfuric acid and iron in reaction tank 1 4 solution, and then the FeSO 4 The concentration of the solution was adjusted to 0.1mol / L. FeSO 4 500ml of the solution and 500ml of the 0.4mol / L NaOH solution are added to the sedimentation tank 2 at the same time, that is, the molar ratio of the actual amount of NaOH used to the amount according to the metering ratio is 2. where FeSO 4 The adding speed of the solution is 5ml / min, and the adding speed of the NaOH solution is 5ml / min. Also add 0.07g tartaric acid, 0.06g ethanol and pass into nitrogen reaction with the flow rate of 5L / min to obtain ferrous hydroxide slurry, after reaction finishes, stop flowing nitrogen. Under the conditions of a temperature of 50° C. and a stirring speed of 800 r / min, air was introduced into the sedimentation tank 2 at a flow rate of 3 L / min to oxidize the prepared ferrous hydroxide slurry. After 1 hour of oxidation, an iron hydroxide slurry was obtained. ...
Embodiment 2
[0036] Prepare FeSO with 1mol / L dilute sulfuric acid and iron in reaction pool 1 4 solution, and then the FeSO 4 The concentration of the solution was adjusted to 0.5mol / L. FeSO 4 Solution 500ml and 1.5L of 1mol / L NaOH solution are added into the sedimentation tank 2 at the same time, that is, the molar ratio of the actual usage amount of NaOH to the amount according to the metering ratio is 3. where FeSO 4 The adding speed of the solution is 3ml / min, and the adding speed of the NaOH solution is 9ml / min. Also add 0.4g sodium dihydrogen phosphate, 0.4g polyethylene glycol and pass into nitrogen reaction with the flow rate of 5L / min to obtain ferrous hydroxide slurry, after the reaction finishes, stop passing through nitrogen. Under the conditions of a temperature of 50° C. and a stirring speed of 900 r / min, air was introduced into the sedimentation tank 2 at a flow rate of 3.5 L / min to oxidize the prepared ferrous hydroxide slurry. After 2 hours of oxidation, an iron hydro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com