Method for producing sodium bicarbonate and sulfur from mirabilite by wet process
A heavy alkali and Glauber's salt technology, applied in the preparation/purification of sulfur, bicarbonate preparation, etc., can solve the problems of increased risk in the process, product use restrictions, etc., and achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
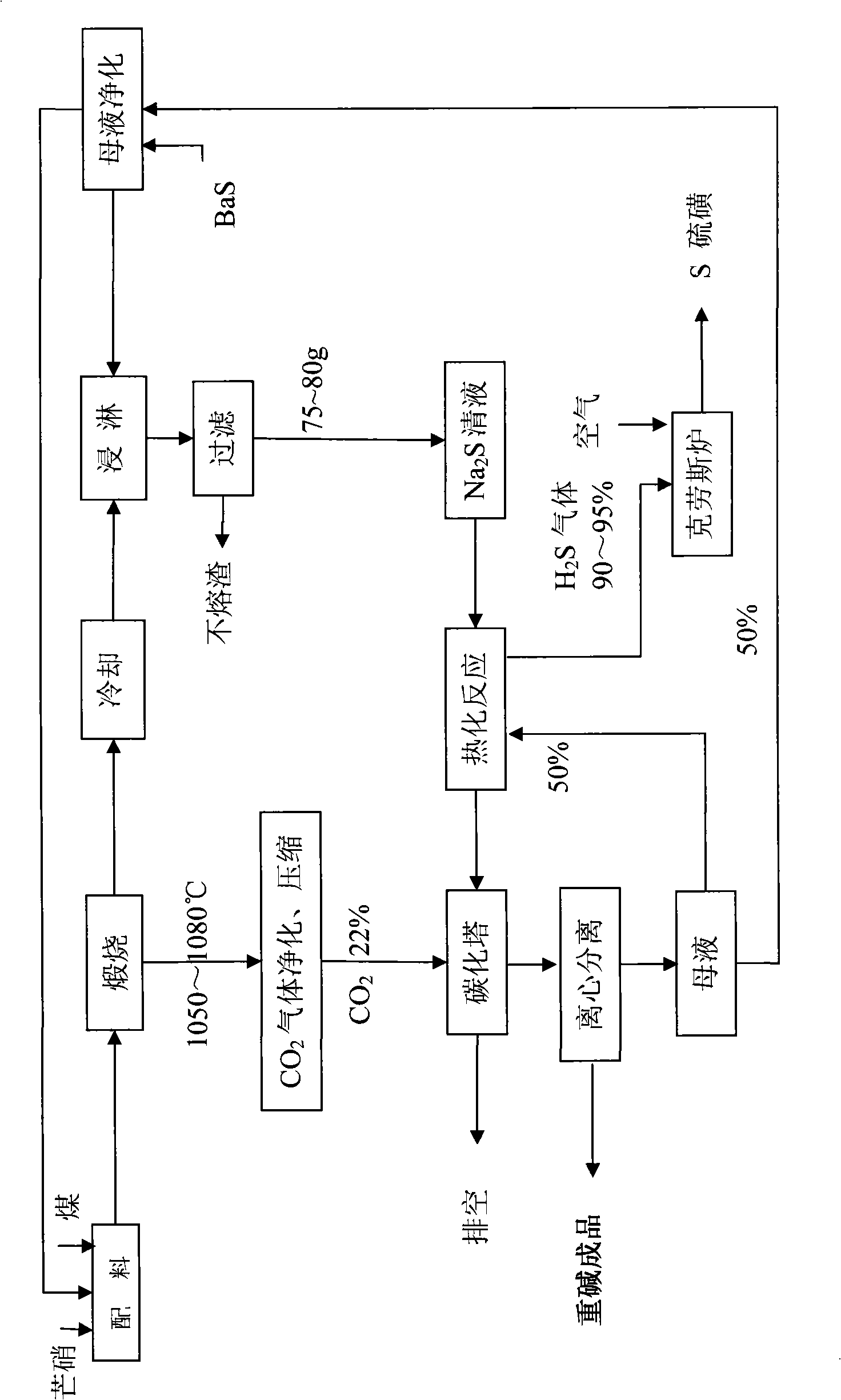
[0045] A. Sodium sulfide preparation process: After mixing 100g of anhydrous Glauber's salt and 24g of coal, put it into porcelain platinum, put it into a muffle furnace and calcinate at 1050°C for 30 minutes, frit 56g, Na 2 The S content is 65%, and it is soaked with 480ml of water after grinding to contain Na 2 S7 5.8 g / l solution.
[0046] B, thermal carbonation process: first in Na 2 Add 95g of heavy alkali to the S clear liquid, boil at 100°C for 5 hours, the released H 2 The concentration of S gas reaches 90%-95%, and the sulfur with a purity of 95-98% is produced by the Claus method; secondly, the boiled solution is naturally cooled to 60-70°C, filtered while it is hot, and the slag is returned to the calcination process;
[0047] C. Sulfur preparation process: use 22% CO for the filtrate 2 The gas is carbonized under the pressure of 0.35MPa, the carbonization temperature is controlled at 50-70°C, and the discharge temperature at the bottom of the tower is controlled...
Embodiment 2
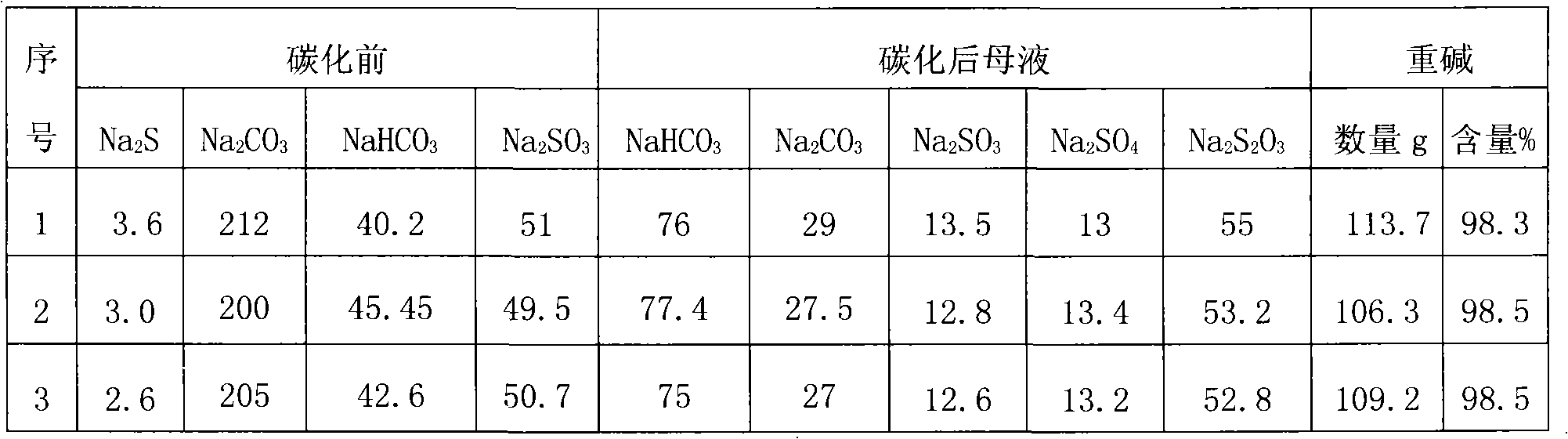
[0050] Embodiment 2: Same as Example 1, the difference is as follows, in the A step soda sulfide preparation process: after mixing with 100g of anhydrous Glauber's salt and 20g of coal, the calcining equipment is a reverberatory furnace, and the calcining temperature is adjusted to 1080°C, and calcined for 60 minutes. Melt block 55g, Na 2 The S content is 64.5%, and it is soaked with 480ml of water after grinding to contain Na 2 S7 3.80 g / l solution. Add 95g of heavy alkali, boil at 105°C for 6h, filter out impurities, and use 22% CO 2 The gas is carbonized under the pressure of 0.35MPa, and the filter cake is dried at 50°C to obtain 106.3g heavy alkali, NaHCO 3 Content 98.5%.
Embodiment 3
[0051] Example 3: Same as Example 2, the difference is as follows, in the step A of alkali sulfide preparation process: after mixing with 100g of anhydrous Glauber's salt and 22g of coal, the calcining equipment is a rotary kiln, and the calcining temperature is adjusted to 1065°C, and calcined for 45 minutes; Add 95g of heavy alkali, boil at 102°C for 3h, filter out impurities, and use 22% CO 2 The gas is carbonized under the pressure of 0.35MPa, and the filter cake is dried at 50°C to obtain 109.2g heavy alkali, NaHCO 3 Content 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com