Clean production process of plateau sulfate type boron-lithium salt lake brine
A sulfate type, salt lake brine technology, applied in lithium sulfate/sulfite, borate, chemical industry, etc., can solve problems such as high environmental protection requirements, no successful examples of comprehensive development of brine, no chemical enterprises, etc., to achieve Widespread effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
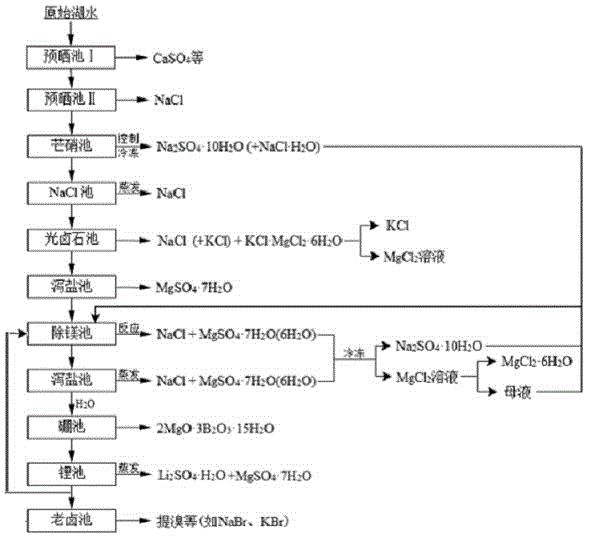
[0043] Example 1 The clean production process of plateau sulfate type boron-lithium salt lake brine includes the following steps (such as figure 1 shown):
[0044] (1) Set up pre-solation pool, Glauber's salt pool, NaCl pool, carnallite pool, Epsom salt pool I, magnesium removal pool, Epsom salt pool II, boron pool, lithium pool and old brine pool.
[0045] (2) Send plateau sulfate-type salt lake brine into the pre-drying pond, and when the mass concentration of sodium ions in the pre-drying pond is controlled to 5%, the plateau sulfate-type salt lake brine is introduced into the Glauber's salt pond; then use winter to precipitate Glauber's salt, and make the liquid The mass concentration of phase sulfate is 0.1%; after the freezing and nitration, brine A is obtained; this brine A is introduced into the NaCl pool; then in spring and summer, the salt is evaporated naturally to obtain brine B; before potassium precipitation, the brine B is introduced into the light brine S...
Embodiment 2
[0054] Example 2 The clean production process of plateau sulfate type boron-lithium salt lake brine comprises the following steps:
[0055] (1) Set up pre-solation pool, Glauber's salt pool, NaCl pool, carnallite pool, Epsom salt pool I, magnesium removal pool, Epsom salt pool II, boron pool, lithium pool and old brine pool.
[0056] ⑵The plateau sulfate-type salt lake brine is sent into the pre-drying tank, and when the mass concentration of sodium ions in the pre-drying tank is controlled to 10%, the plateau sulfate-type salt lake brine is introduced into the Glauber's salt tank; The mass concentration of phase sulfate is 0.5%; after the freezing and nitration, brine A is obtained; this brine A is introduced into the NaCl pool; then in spring and summer, the salt is evaporated naturally to obtain brine B; before potassium precipitation, the brine B is introduced into light brine Stone pool.
[0057] Among them: one or two pre-drying ponds.
[0058] (3) The natural eva...
Embodiment 3
[0065] Example 3 The clean production process of plateau sulfate type boron-lithium salt lake brine comprises the following steps:
[0066] (1) Set up pre-solation pool, Glauber's salt pool, NaCl pool, carnallite pool, Epsom salt pool I, magnesium removal pool, Epsom salt pool II, boron pool, lithium pool and old brine pool.
[0067] ⑵The plateau sulfate-type salt lake brine is sent into the pre-drying pond, and when the mass concentration of sodium ions in the pre-drying pond is controlled to 7%, the plateau sulfate-type salt lake brine is introduced into the Glauber's salt pond; The mass concentration of sulfate radicals is 0.3%; after freezing and nitrating, brine A is obtained; this brine A is imported into the NaCl pool; then in spring and summer, salt is evaporated naturally to obtain brine B; before potassium precipitation, brine B is introduced into light brine Stone pool.
[0068] Among them: one or two pre-drying ponds.
[0069] (3) The natural evaporation of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com