Methods for coproducing sodium carbonate and ammonium sulfate from melamine tail gas and mirabilite
A technology of ammonium sulfate and soda ash, applied in the direction of ammonium sulfate and carbonate preparations, etc., can solve the problem that melamine tail gas cannot be fully utilized as waste heat and steam, and achieve the expansion of large-scale utilization methods, obvious energy saving and consumption reduction, and economic benefits. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
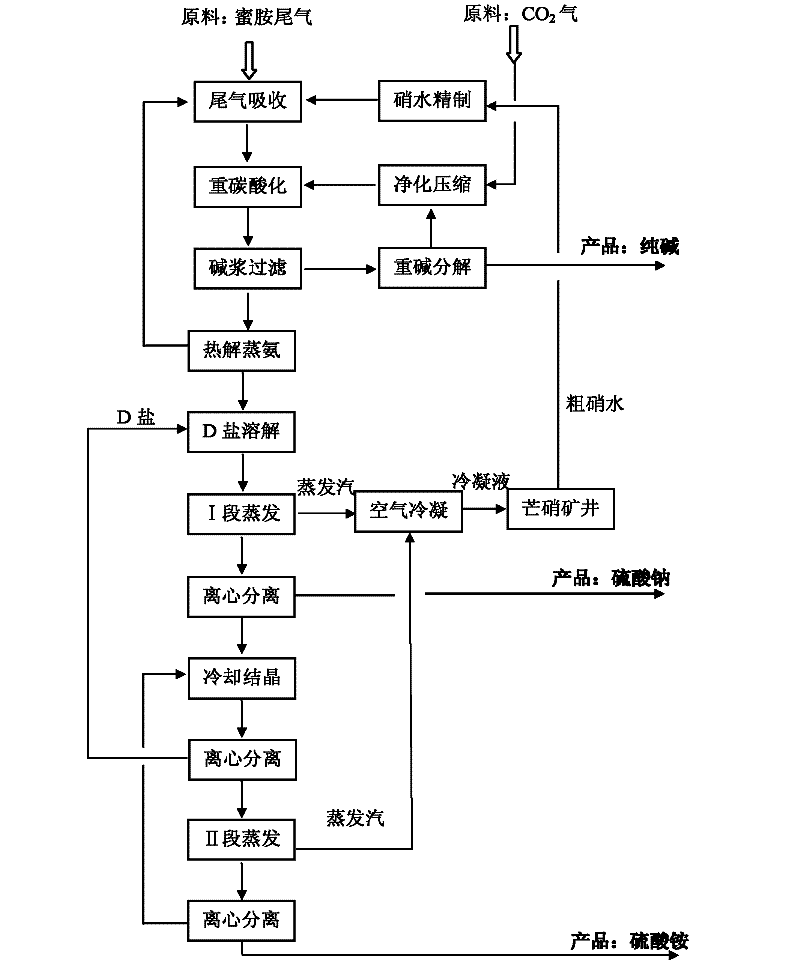
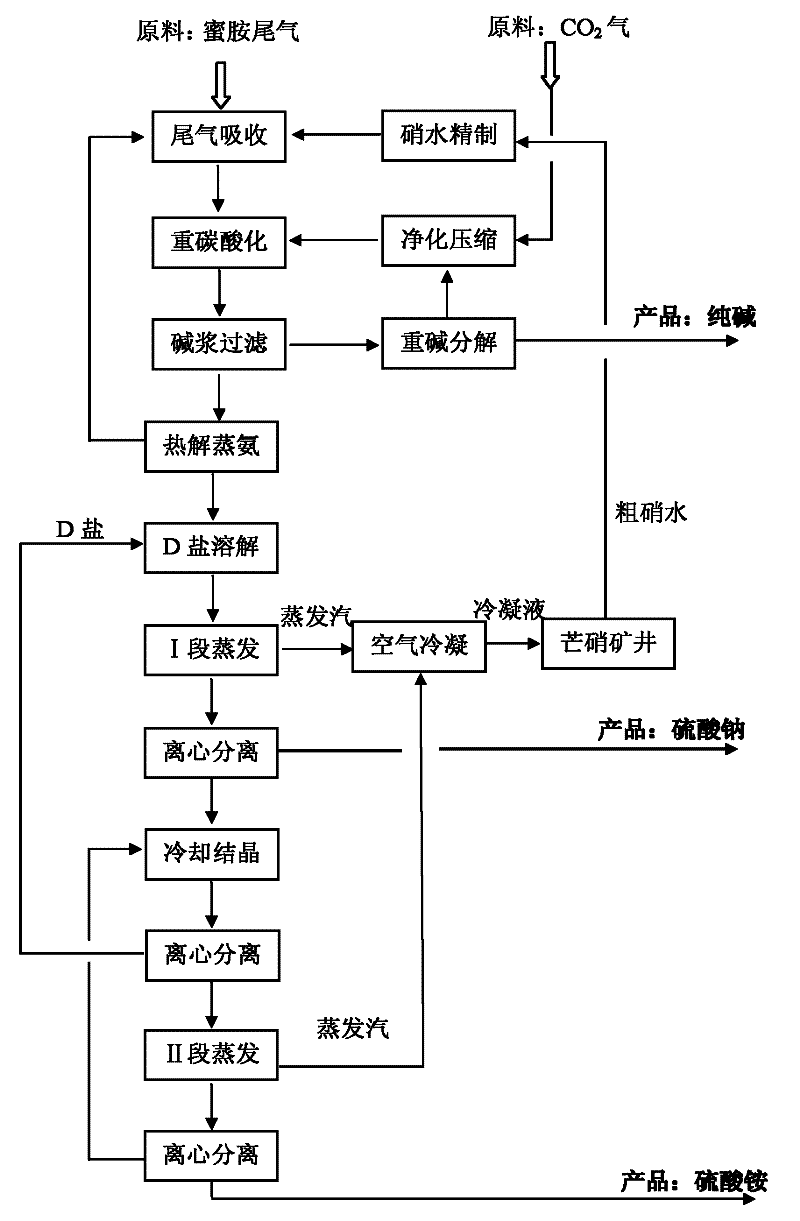
[0143] refer to figure 1 , Embodiment 1 of the present invention will be described in detail.
[0144] Join Na 2 CO 3 and NaOH, the crude Glauber's salt aqueous solution is refined by the two-alkali method, and the above-mentioned crude Glauber's salt aqueous solution is treated so that the total concentration of calcium and magnesium ions is lower than 10ppm and Na 2 SO 4 A refined sodium sulfate aqueous solution with a content of 29 wt%.
[0145] With the above Na 2 SO 4 The 29wt% refined sodium sulfate aqueous solution absorbs the tail gas of the melamine process, the temperature is controlled at 42-45°C to ensure the complete absorption of the melamine tail gas, and the amount of the refined sodium sulfate aqueous solution is adjusted to make an ammonium carbonate-sodium sulfate aqueous solution containing 9.5% ammonia . Continue to feed CO into the ammonium carbonate-sodium sulfate aqueous solution 2 Gas, control the reaction temperature to be 65°C, so that the a...
Embodiment 2
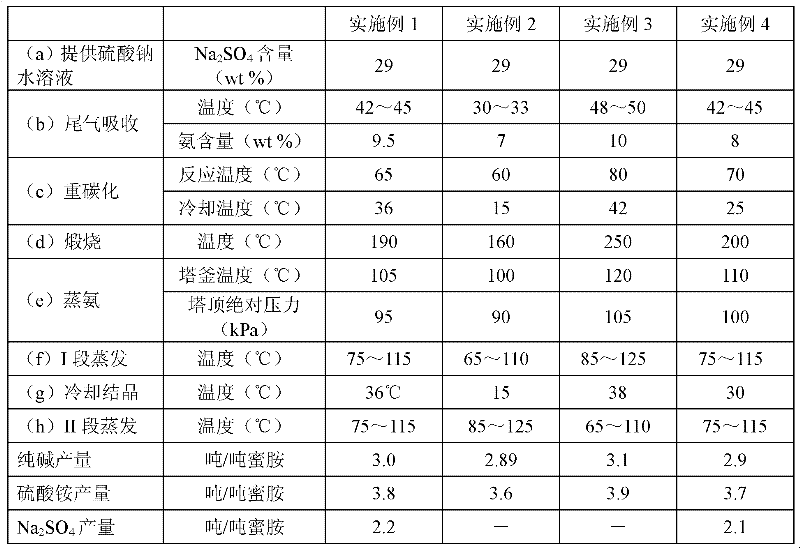
[0153] Embodiment 2 is carried out according to the conditions shown in table 1, Na 2 SO 4 The filter cake is returned to the alkali-making process as a raw material, and other steps and conditions are the same as in Example 1.
Embodiment 3
[0155] Embodiment 3 is carried out according to the conditions shown in table 1, Na 2 SO 4 The filter cake is returned to the alkali-making process as a raw material, and other steps and conditions are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com