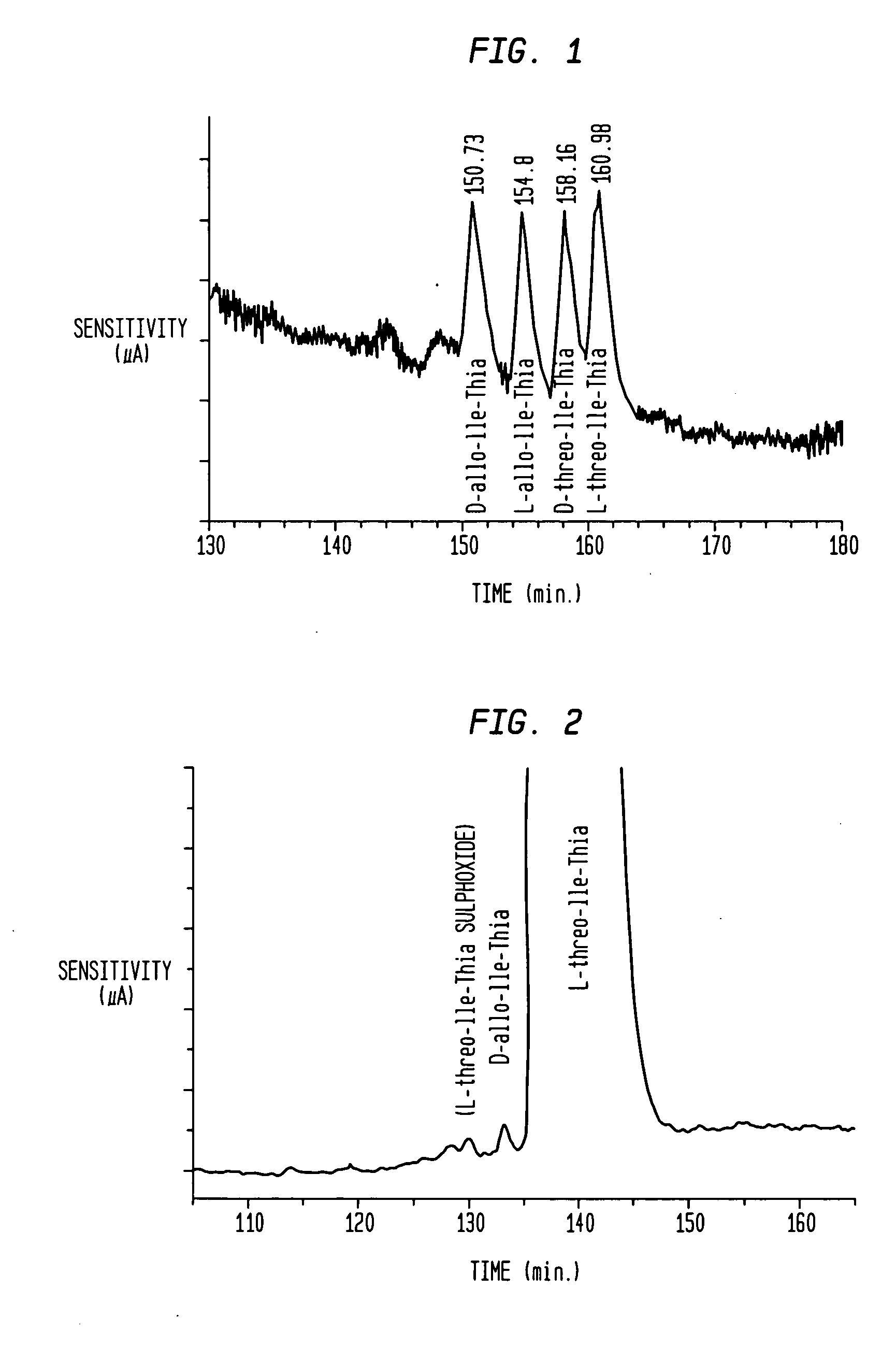
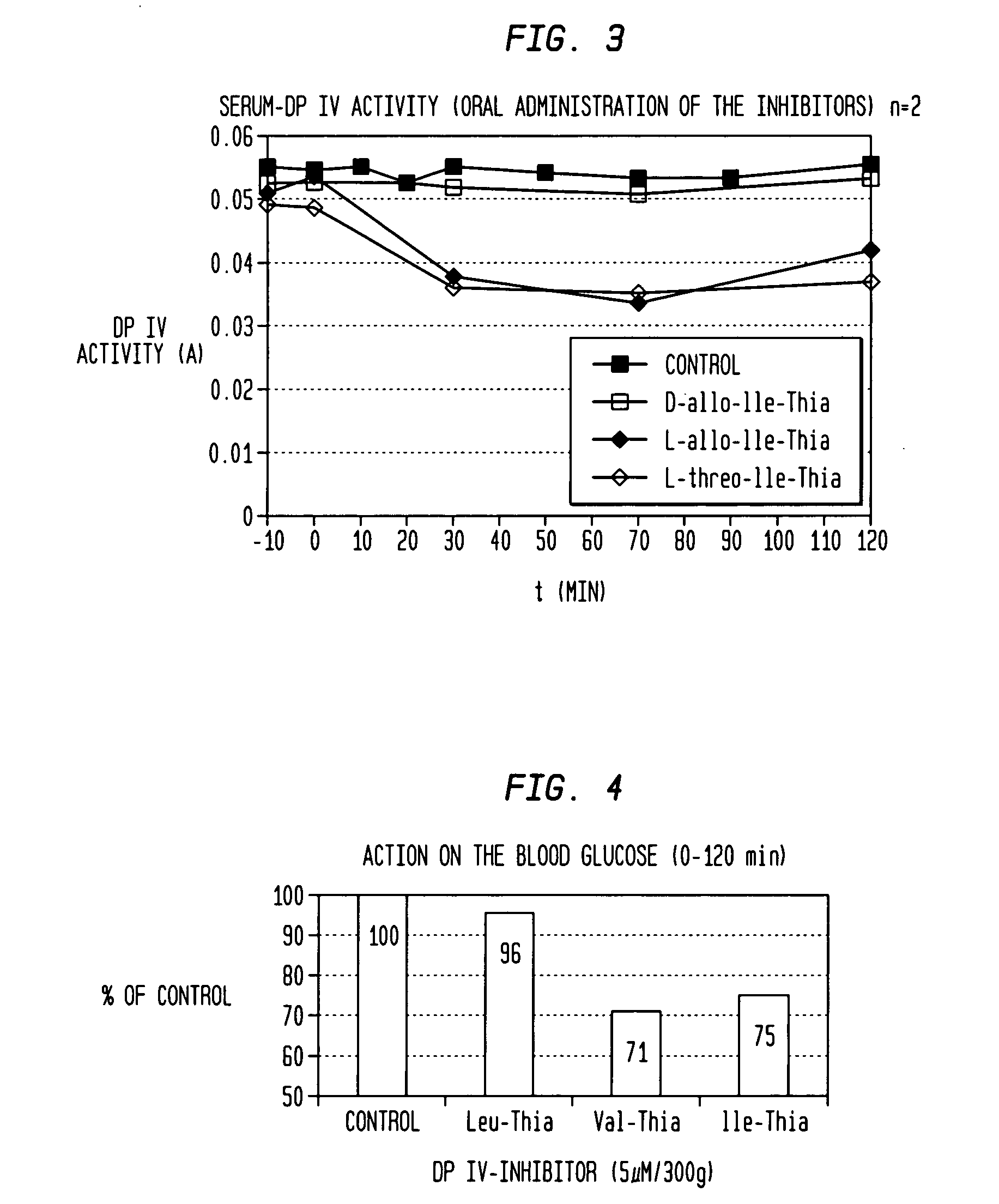
Novel effectors of dipeptidyl peptidase IV
a technology of dipeptidyl peptidase and effectors, which is applied in the field of new effectors of dipeptidyl peptidase iv, can solve the problems of serious vascular changes, high cost, and distinct impairment of the quality of life of patients, and achieve the effect of simple treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] On administration, preferably oral administration, of these effectors to a mammalian organism, the endogenous (or additionally exogenously administered) insulinotropic peptides GIP1-42 and GLP-17-36 (or alternatively GLP-17-37 or analogues thereof) are broken down to a reduced extent by DP IV or DP IV-like enzymes and therefore the decrease in the concentration of those peptide hormones or their analogues is reduced or delayed. The invention is therefore based on the finding that a reduction in the DP IV or DP IV-like enzymatic activity acting in the blood circulation has an effect on the blood sugar level. It has been found that [0019] 1. the reduction in DP IV or DP IV-analogous activity leads to an increase in the relative stability of the glucose-stimulated or externally introduced incretins (or analogues thereof), that is to say by administration of effectors of DP IV or DP IV-analogous proteins it is possible to control the breakdown of incretin in the blood; [0020] 2. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com