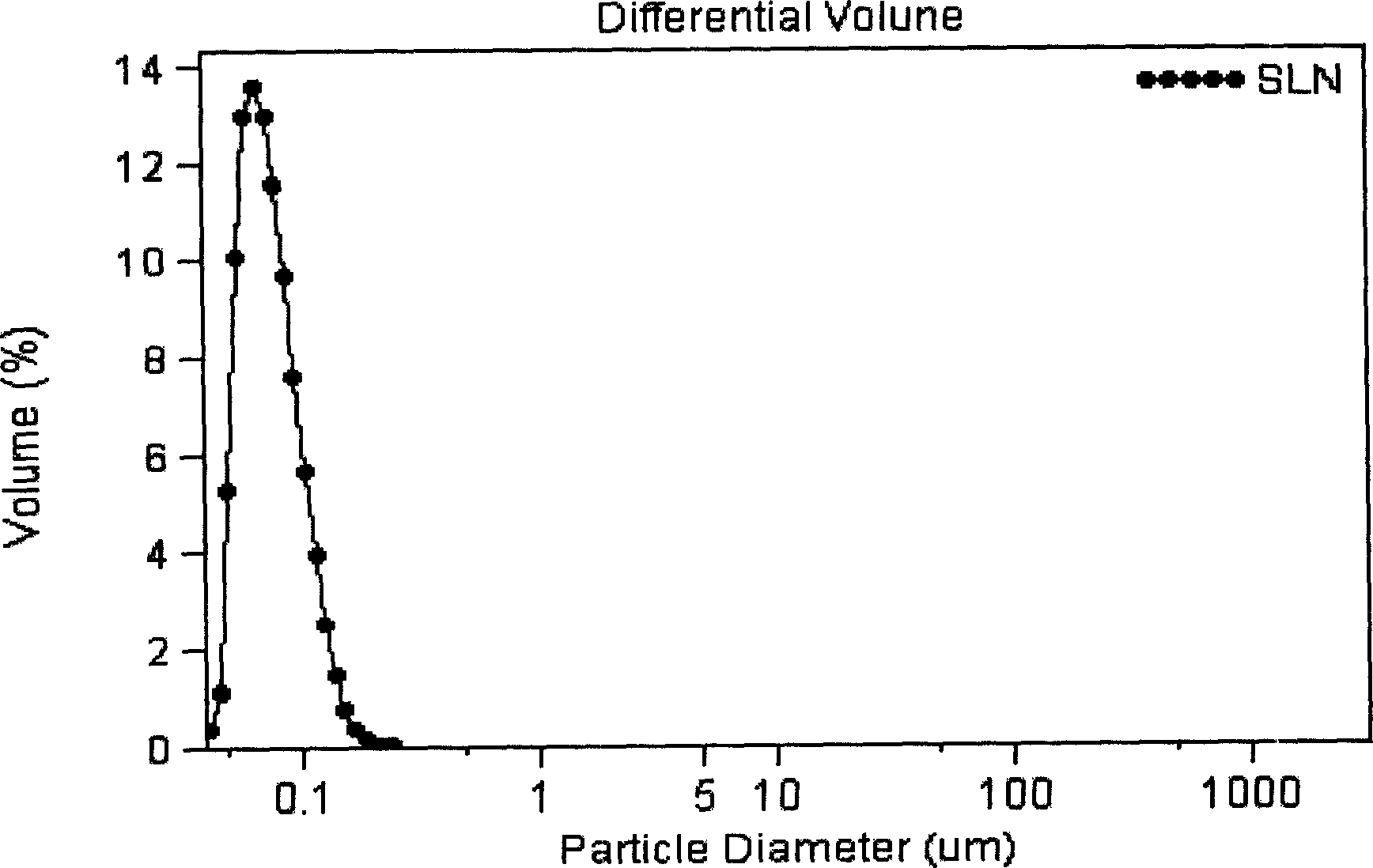
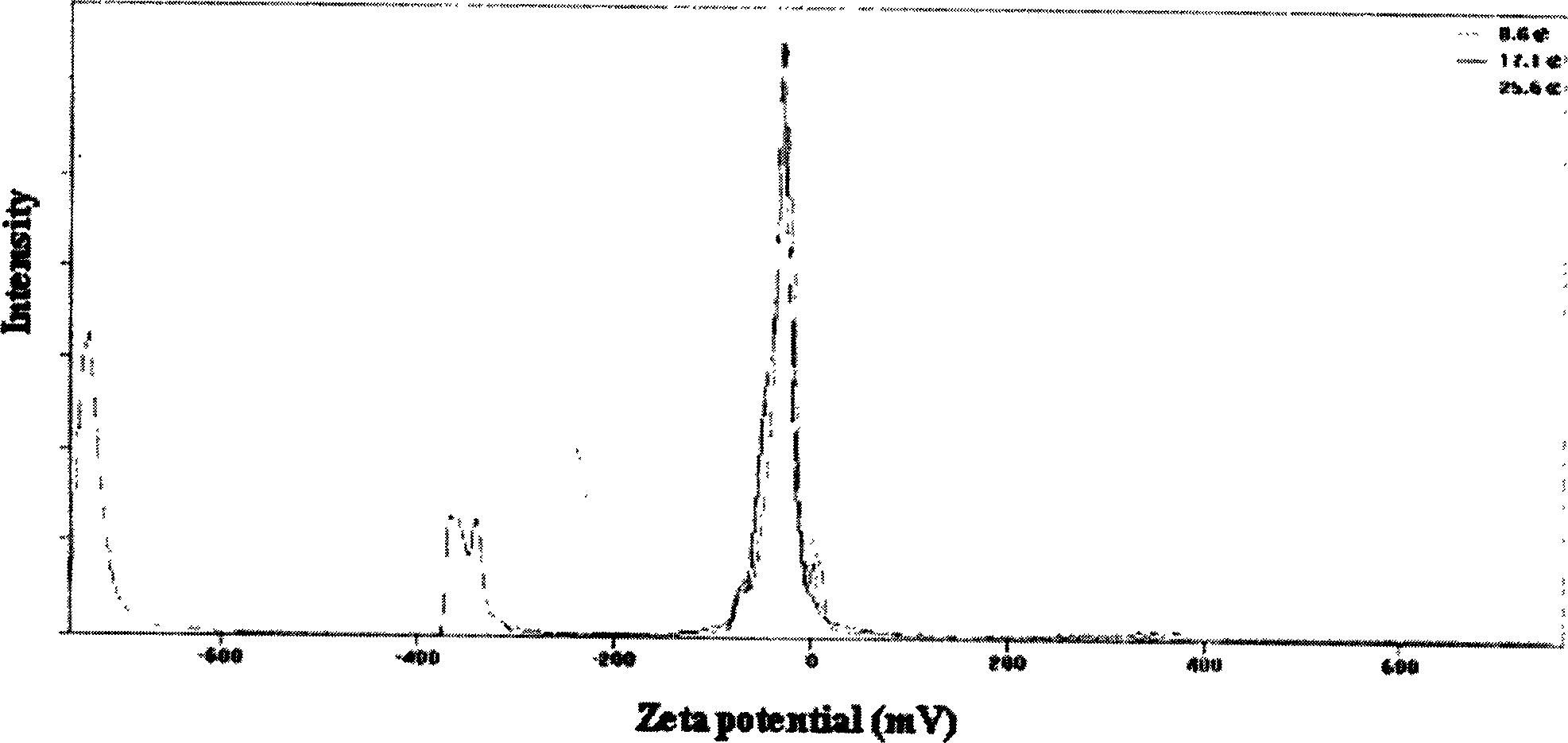
Vinpocetine solid liposome nano-particle and its preparation technology
A technology of solid lipid nanometer and vinpocetine, which is applied in liposome delivery, cardiovascular system diseases, nervous system diseases, etc., and can solve problems such as low absolute bioavailability and liver first-pass effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Prescription: Vinpocetine 5mg
[0043] Phospholipids 100-300mg
[0044] Poloxamer-188 100-300mg
[0045] Glyceryl monostearate 150-400mg
[0046] Water 10-20mL
[0047] Preparation method: ①Dissolve vinpocetine in a hot solution containing glyceryl monostearate, stir evenly, add the aqueous solution containing phospholipids and poloxamer 188 dropwise into the oil phase under gentle stirring to make a microemulsion.
[0048] ②Under mild mechanical mixing conditions, the microemulsion is dispersed in a cold water phase medium (2-3° C.) to form a solid lipid nanoparticle suspension.
Embodiment 2
[0050] Prescription: Vinpocetine 5mg
[0051] Phospholipids 100-300mg
[0052] Polyoxyethylene hydrogenated castor oil 100-300mg
[0053] Glyceryl Tristearate 150-400mg
[0054] Water 10-20mL
[0055] Preparation method: ① Dissolve vinpocetine in a hot solution containing glyceryl tristearate, stir evenly, add the aqueous solution containing phospholipids and Cremophor RH 40 into the oil phase and mix well to form colostrum, then crush the cells machine ultrasound.
[0056] ②Through the homogenizer cycle and homogenize for 3-5 times, cool down to form a solid lipid nanoparticle suspension.
Embodiment 3
[0058] Prescription: Vinpocetine 5mg
[0059] Phospholipids 100-300mg
[0060] Tween-80 100-300mg
[0061] Glyceryl Behenate 150-400mg
[0062] Water 10-20mL
[0063] Preparation method: ① Dissolve vinpocetine in a hot solution containing glyceryl behenate, stir evenly, add the aqueous solution containing phospholipids and Tween-80 into the oil phase and fully mix it into colostrum, then pass through a cell pulverizer ultrasound.
[0064]②After cooling, the solid lipid nanoparticle suspension is formed.
[0065] ③ Add freeze-dried support agent (trehalose, mannitol, dextran, sorbitol, lactose, sucrose, etc.) to prepare freeze-dried powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com