Preparation technology of high-purity vinpocetine
A vinpocetine and preparation technology, which is applied in the field of preparation of high-purity vinpocetine, can solve the problems of limited product purity, serious environmental pollution, equipment corrosion, etc., achieve ideal yield and purity, simple operation process, reduce Effects of Environmental Pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
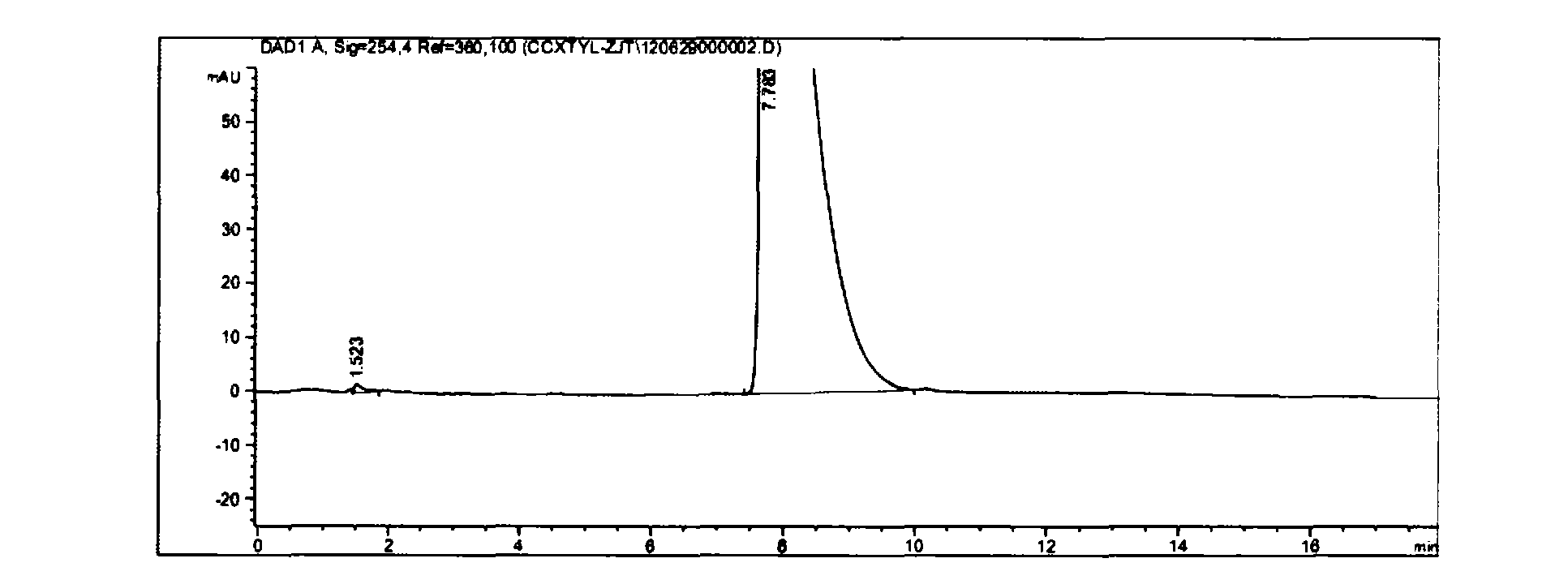
[0037] At room temperature, mix 3g of vincamine and 8ml of DMF and stir evenly, cool to 0-5 degrees, stir, and slowly add 0.96ml of SOCl dropwise 2 Into the reaction bottle (strictly control the reaction temperature not exceeding 30 degrees), react for 3 hours (room temperature 20-25 degrees), TLC and HPLC follow the reaction. After the raw materials disappear, cool to 0-5 degrees, add 250ml of ice water to quench, then cool to 0-5 degrees, add ammonia water (content 25%-28%) 83ml to adjust to PH=8 (strictly control the reaction temperature not to exceed 25 degree), a solid was precipitated, stirred within 10 degrees for half an hour, suction filtered, washed with a large amount of water, and washed with a small amount of ethanol to obtain 2.74 g of the intermediate Apovincamin. (Yield = 96%, HPLC: 99.8%)
Embodiment 2
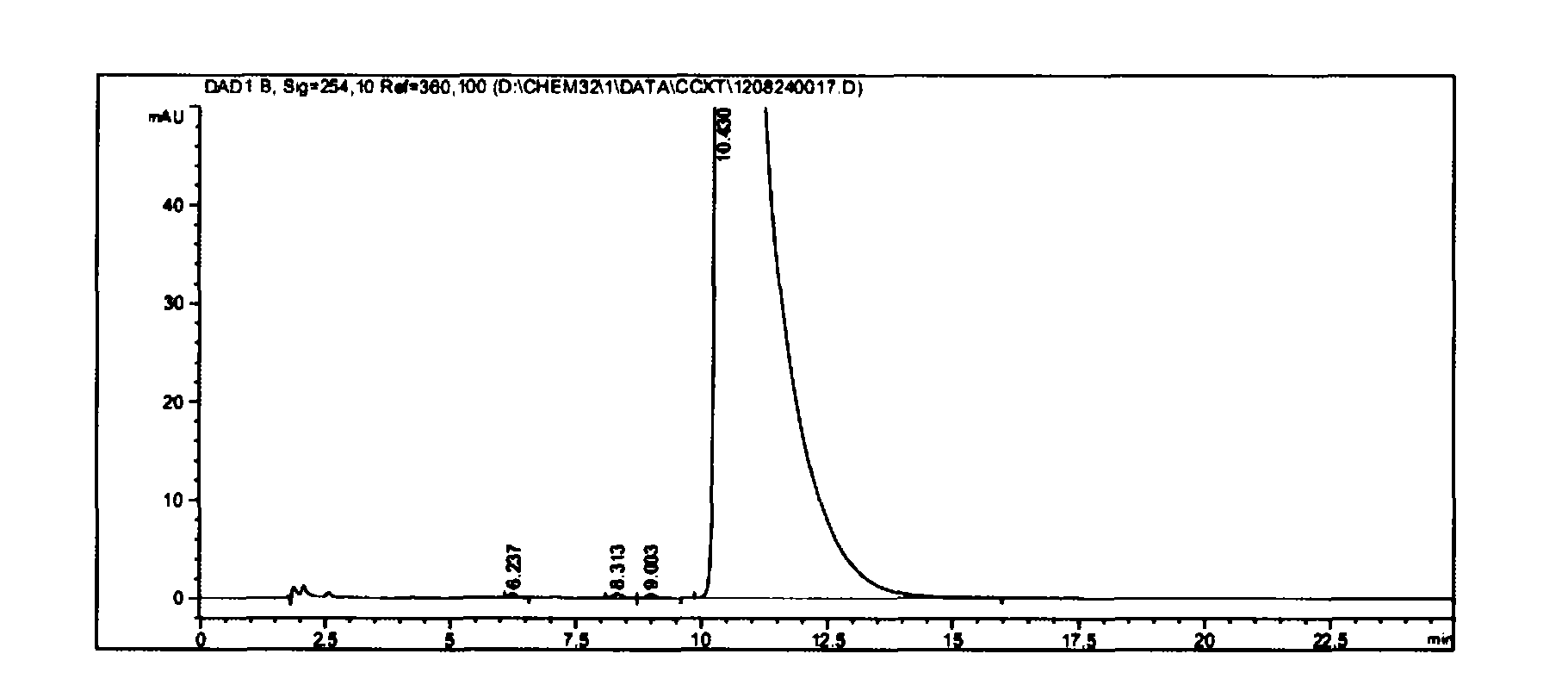
[0039] At room temperature, mix and stir vincamine 3g and 7.2ml DMF evenly, cool to 0-5 degrees, keep stirring, slowly add 0.84ml SOCl dropwise 2 Into the reaction bottle (strictly control the reaction temperature not exceeding 30 degrees), react for 1 hour (room temperature 20-25 degrees), TLC and HPLC follow the reaction. After the raw materials disappear, cool to 0-5 degrees, add 15ml of ice water to quench (strictly control the reaction temperature not to exceed 25 degrees), then cool to 0-5 degrees, add ammonia water (content 25% to 28%) to adjust to PH = 8 (strictly control the reaction temperature not to exceed 25 degrees), precipitate solids, stir within 10 degrees for half an hour, filter with suction, wash with a large amount of water, and wash with a small amount of ethanol to obtain 2.74 g of the intermediate Apovincamin. (Yield = 96%, HPLC: 99.6%)
Embodiment 3
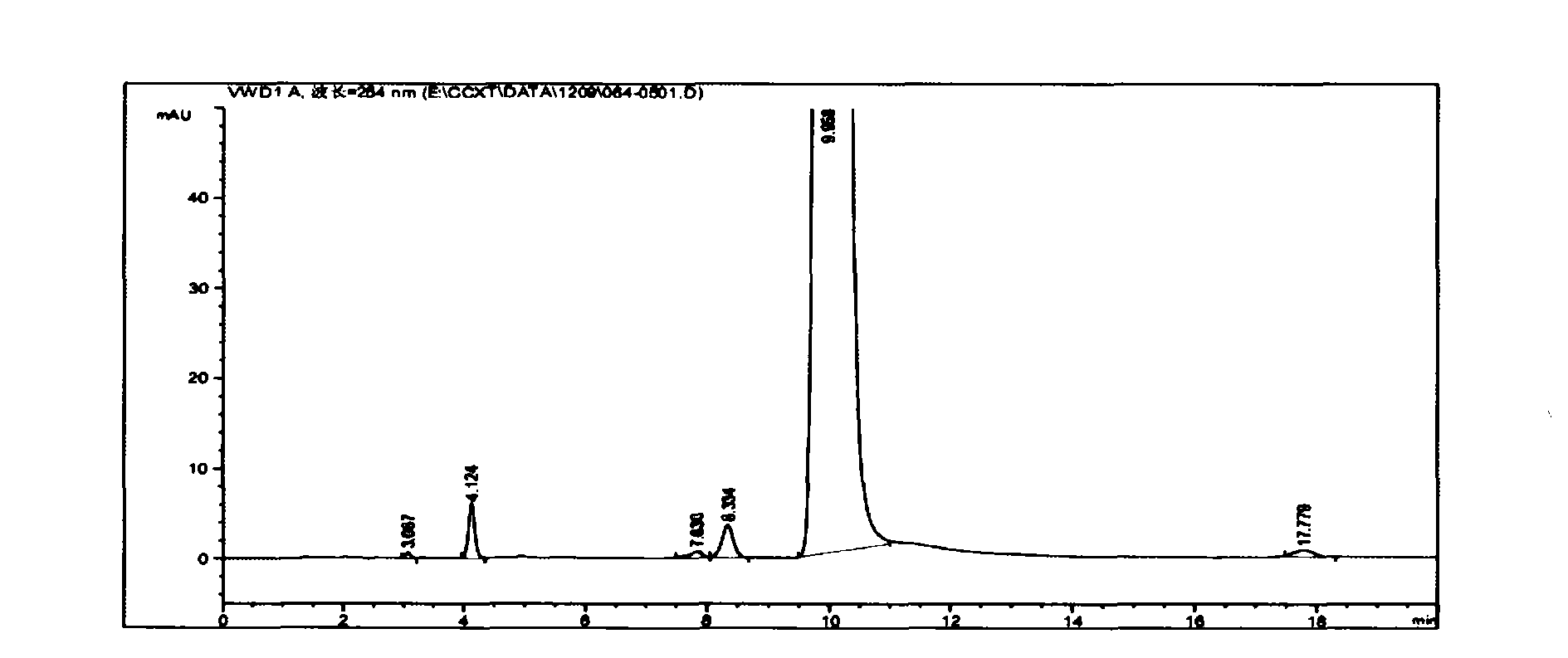
[0041] At room temperature, mix and stir 3g vincamine and 7.2ml DMF evenly, cool to 0-5 degrees, keep stirring, slowly add 0.66ml SOCl dropwise 2 Into the reaction bottle (strictly control the reaction temperature not exceeding 30 degrees), react for 1 hour (room temperature 20-25 degrees), TLC and HPLC follow the reaction. After the raw materials disappear, cool to 0-5 degrees, add 15ml of ice water to quench (strictly control the reaction temperature not to exceed 25 degrees), then cool to 0-5 degrees, add ammonia water (content 25%-28%) to adjust to PH = 8 (strictly control the reaction temperature not to exceed 25 degrees), precipitate solids, stir within 10 degrees for half an hour, filter with suction, wash with a large amount of water, and wash with a small amount of ethanol to obtain 2.74 g of the intermediate Apovincamin. (Yield = 96%, HPLC: 99.5%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com