Vinpocetine freeze-dried powder for injection and preparation thereof
A technology for freeze-dried powder injection and vinpocetine, applied in the field of medicine, can solve the problems of toxic and side effects, poor stability of vinpocetine freeze-dried powder injection, etc., and achieve the effects of stable physical and chemical properties, good stability and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
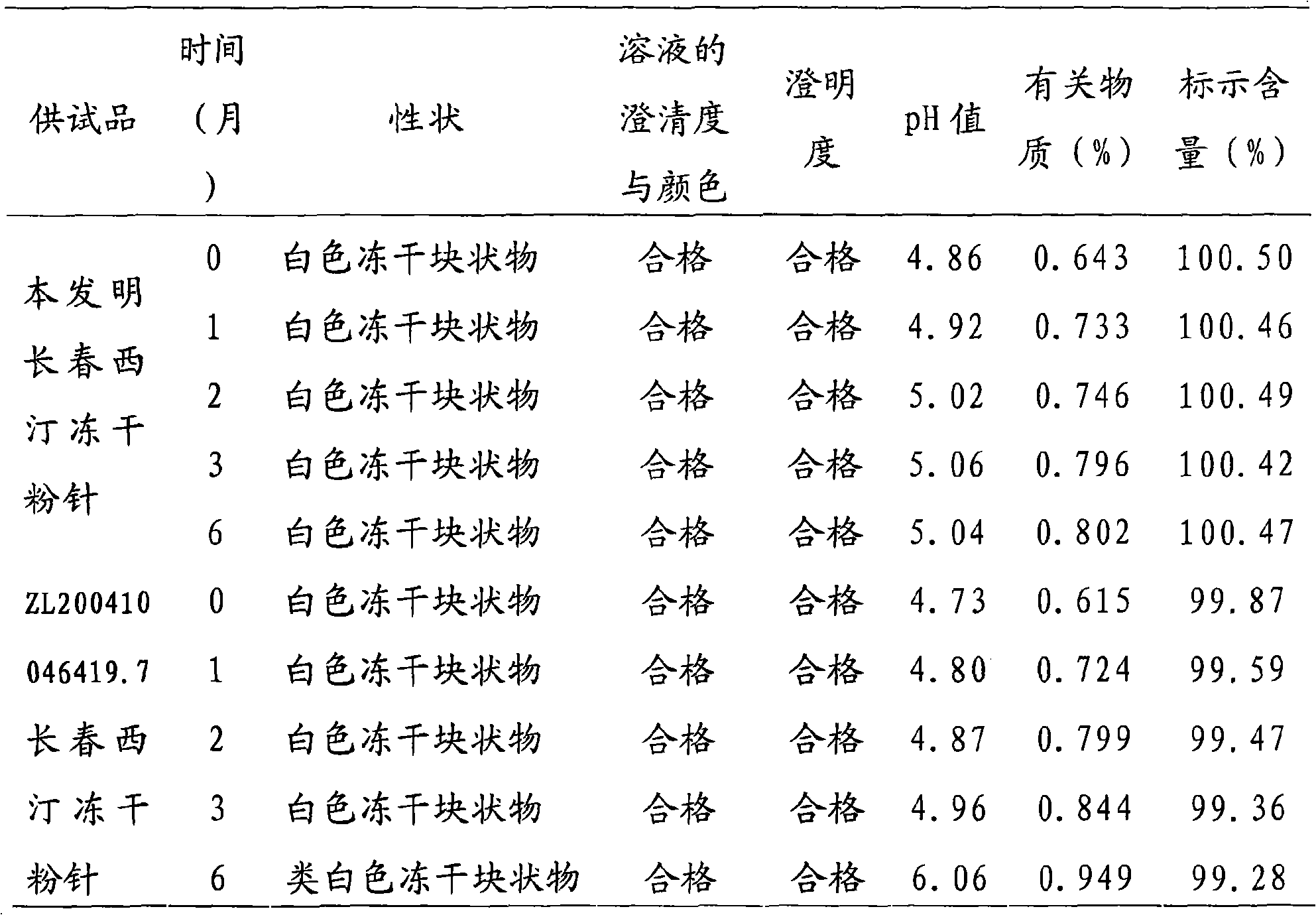
Examples
Embodiment 1
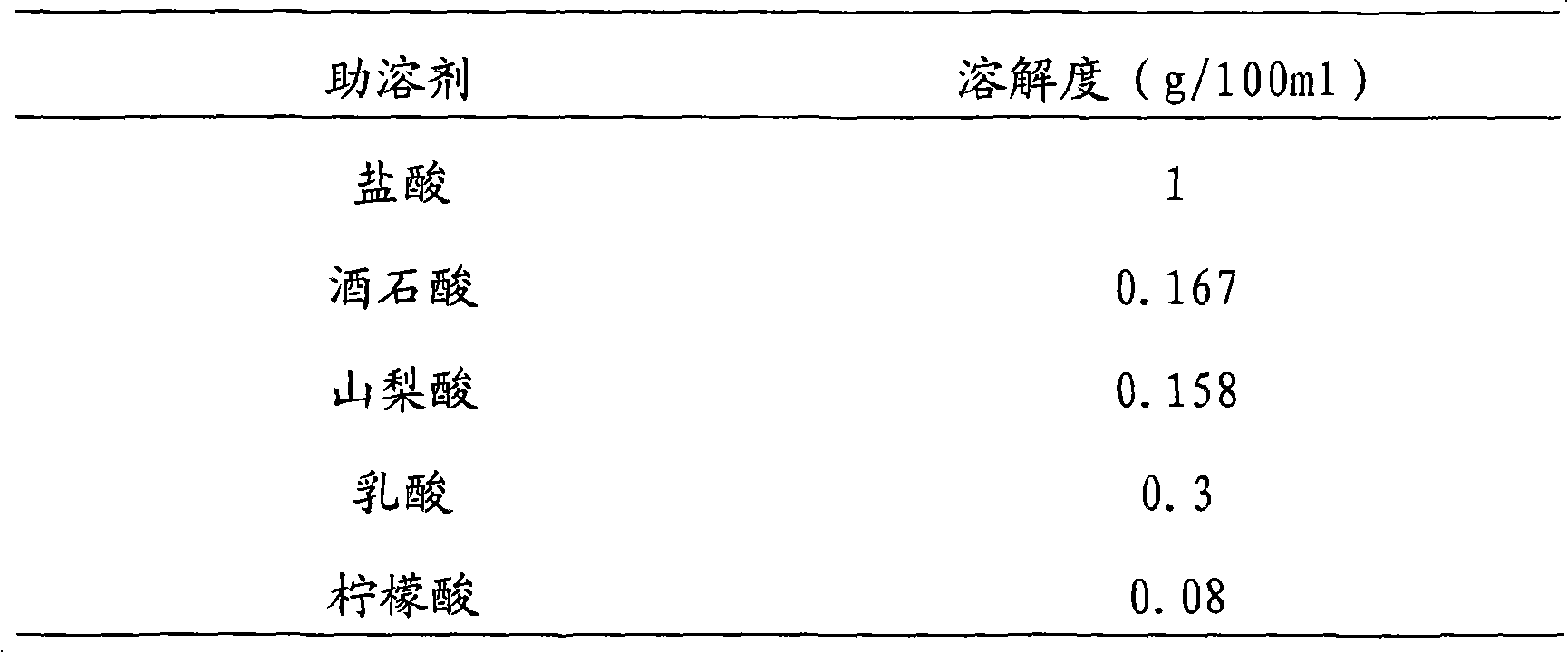
[0024] The prescription is:
[0025] Vinpocetine 10g
[0026] Mannitol 90g
[0027] 1mol / L hydrochloric acid 50ml
[0028] Add water for injection to 2000ml
[0029]
[0030] A total of 1000 sticks were prepared
[0031] The preparation process is:
[0032] 1) Aseptic treatment of glass bottles, rubber stoppers and aluminum caps.
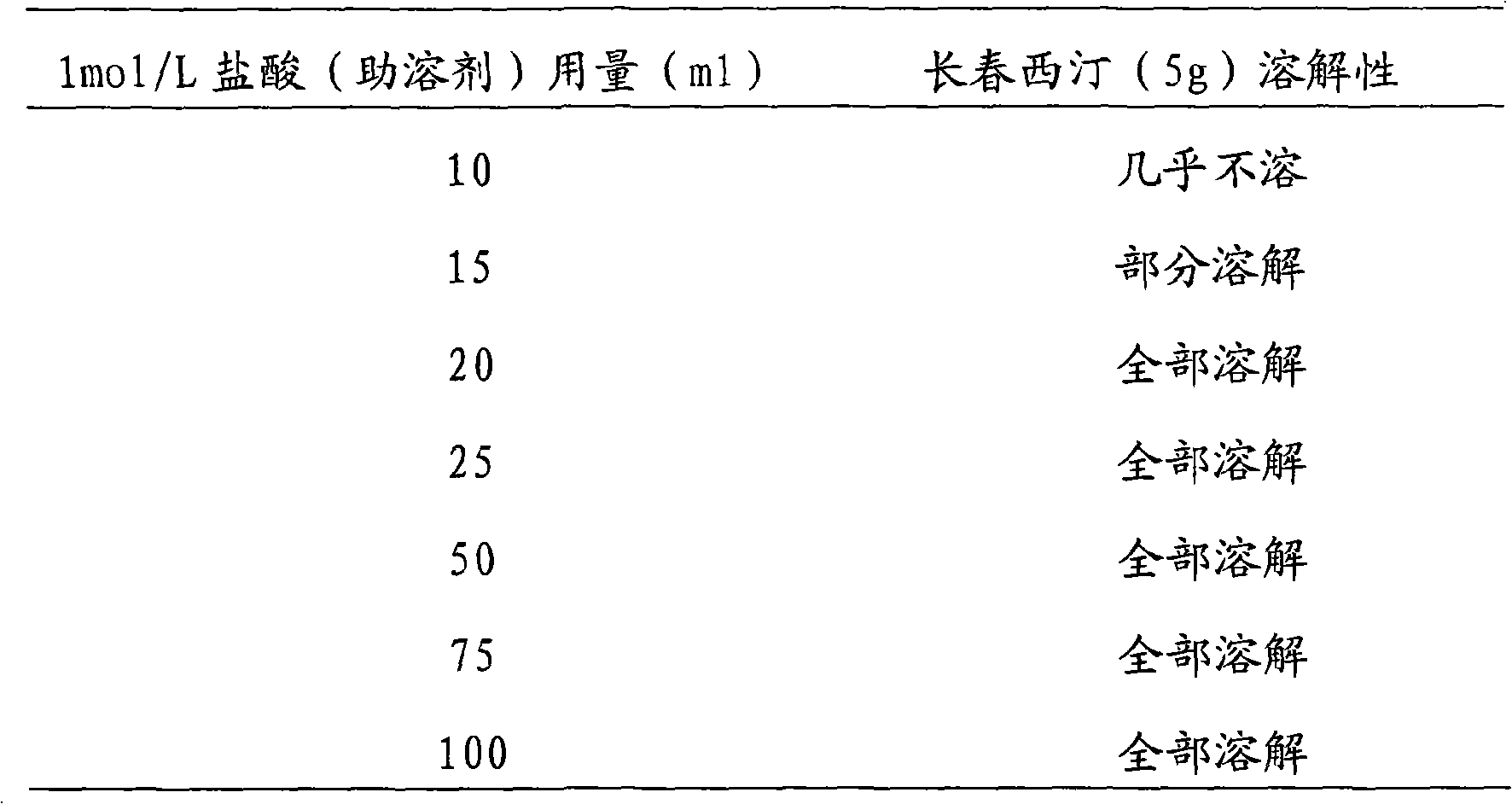
[0033] 2) Weigh the raw and auxiliary materials, add the mannitol into the sterile water for injection, stir to dissolve. Add vinpocetine into 50ml of 1mol / L hydrochloric acid solution, stir to dissolve. Mix the two solutions, add water for injection to the full amount, and stir evenly.
[0034] 3) Adjust the pH value of the solution to 3.5 with 0.5 mol / L hydrochloric acid solution.
[0035] 4) Add 0.1% activated carbon for needles to the prepared solution, heat and stir for 20 minutes, let stand for 20 minutes, and remove carbon by suction filtration.
[0036] 5) Fine filter with a sterile microporous membran...
Embodiment 2
[0041] The prescription is:
[0042] Vinpocetine 20g
[0043] Mannitol 180g
[0044] 1mol / L hydrochloric acid 100ml
[0045] Add water for injection to 5000ml
[0046]
[0047] A total of 2000 sticks were prepared
[0048] The preparation process is:
[0049] 1) Aseptic treatment of glass bottles, rubber stoppers and aluminum caps.
[0050] 2) Weigh the raw and auxiliary materials, add mannitol into sterile water for injection and stir to dissolve. Add vinpocetine into 100ml of 1mol / L hydrochloric acid solution, stir to dissolve. Mix the two, add water for injection to the full amount, and stir evenly.
[0051] 3) Adjust the pH value of the solution to 4.5 with 1mol / L hydrochloric acid solution.
[0052] 4) Add 0.1% activated carbon for needles to the prepared solution, heat and stir for 20 minutes, let stand for 20 minutes, and remove carbon by suction filtration.
[0053] 5) Fine filter with a sterile microporous membrane (0.22 μm), an...
Embodiment 3
[0058] The prescription is:
[0059] Vinpocetine 30g
[0060] Mannitol 250g
[0061] 1mol / L hydrochloric acid 150ml
[0062] Add water for injection to 5000ml
[0063]
[0064] A total of 3000 sticks were prepared
[0065] The preparation process is:
[0066] 1) Aseptic treatment of glass bottles, rubber stoppers and aluminum caps.
[0067] 2) Weigh the raw and auxiliary materials, add the mannitol into the sterile water for injection, stir to dissolve. Vinpocetine was added into 150ml of 1mol / L hydrochloric acid solution, stirred to dissolve. Mix the two, add water for injection to the full amount, and stir evenly.
[0068] 3) Adjust the pH value of the solution to 4.0 with 2mol / L hydrochloric acid solution.
[0069] 4) Add 0.1% activated carbon for needles to the prepared solution, heat and stir for 20 minutes, let stand for 20 minutes, and remove carbon by suction filtration.
[0070] 5) Fine filter with a sterile microporous membrane (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com