Method of detecting apovincamine acid and vincamine acid in vinpocetine simultaneously
A technology of apo-vinblastine and vinpocetine is applied in the field of analytical chemistry to achieve the effects of high peak symmetry, high system adaptability and high precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 1. Instruments and reagents
[0045] (1) LC-20AD high performance liquid chromatograph (Japan Shimadzu company), including LC-20AD pump, SPD-M20A diode array detector (190nm~800nm), SIL-20A automatic sampler, Labsolution workstation; E2695 high performance liquid chromatograph (U.S. Waters company), including 2998 diode array detector (190nm ~ 800nm), Empower3 workstation; AUW 220D double range analytical balance (sensitivity 0.1mg / 0.01mg, Japan Shimadzu company); SHH-100GD-2 drug strong light irradiation test chamber (Chongqing Yongsheng Experimental Instrument Factory); GXZ-9240 MBE blast drying oven (Shanghai Boxun Industrial Co., Ltd. Medical Equipment Factory)
[0046] (2) Reagents: Except for methanol, which is chromatographic grade, other reagents are analytically pure.
[0047] (3) Reference substance: Vinpocetine reference substance (CAS: 42971-09-5), batch number: 100947-201203, provided by China National Institutes for Food and Drug Control. Apvincine refer...
experiment example 1
[0078] Experimental Example 1 System Suitability Test and Interference Test
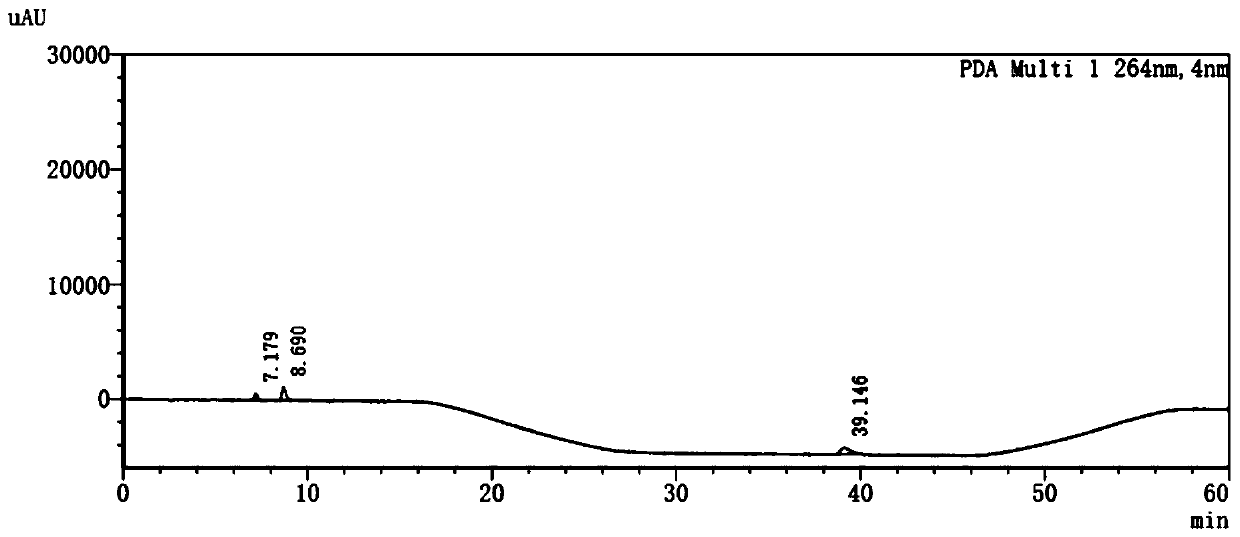
[0079] 1. Take 10 μl of the system suitability solution and inject it into the liquid chromatograph, and record the chromatogram. The system suitability solution: take an appropriate amount of vinpocetine, vinblastine and apovincine acid reference substance, add mobile phase A- Methanol (60:40) was dissolved and diluted to make a mixed solution containing about 1 μg of vinpocetine, 0.5 μg each of vincine and apovincine per 1 ml, as a system suitability solution.
[0080] Table 4 System Suitability Results
[0081] Compound name concentration retention time (min) Separation Vinpocetine 1μg / ml 39.146 43.9 vinblastine 0.5μg / ml 7.179 / Apovincine 0.5μg / ml 8.690 4.2
[0082] The result is as figure 1 As shown, the order of the peaks is vinblastine, apovincine, and vinpocetine. The main peak retention time of vinpocetine is 39.146 minutes, and the peak retent...
experiment example 2
[0085] Experimental example 2 Forced degradation test
[0086] In order to further investigate the impact of impurities or degradation products that may exist in the sample on the determination of vincine and apovincine, this method carried out a forced degradation test. Through strong light irradiation, high temperature, acid-base hydrolysis, oxidation and other methods, the destruction of vinpocetine and vinpocetine injection is accelerated, and the separation of degradation products and unknown impurities of samples from vinblastine and apovincine acid is investigated. , to evaluate the validity and applicability of the method.
[0087] 1. Forced degradation test of vinpocetine raw material
[0088] Under the conditions of light and high temperature destruction, vinpocetine was basically not degraded, and no vincine and apovincine were detected in the suspension and powder destruction samples, and the stability was good. Under the conditions of oxidation, alkali, and acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com