Medicament for treating cerebral blood-vessel dilate and preparation method thereof
A cerebrovascular and vinpocetine technology, applied in the field of pharmaceutical preparations, can solve problems such as poor water solubility, affecting drug absorption and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1 (by 1000 bottles)
[0086] Take the raw and auxiliary materials by the following parts by weight:
[0087] 10 parts by weight of vinpocetine, 1 part by weight of vitamin C, 2 parts by weight of sodium metabisulfite, 200 parts by weight of mannitol, 100 parts by weight of sorbitol, and 4 parts by weight of tartaric acid.
[0088] Above-mentioned dosage form preparation method of the present invention is as follows:
[0089] 1. Take freshly prepared water for injection, boil it, wait until the temperature drops to 40°C, and set aside.
[0090] 2. Measure 1600ml of the above-mentioned water for injection, add vitamin C, stir to dissolve, slowly add vinpocetine, after dissolving, add sodium metabisulfite, sorbitol, mannitol, and tartaric acid to dissolve.
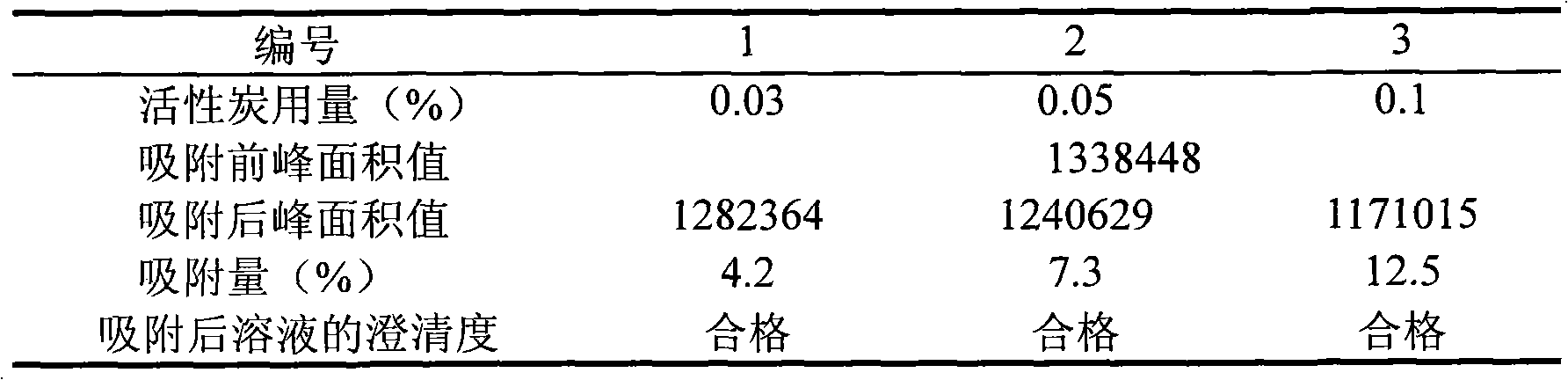
[0091] 3. Stir well, measure the pH value, adjust the pH value to 3.5 (3.0-4.0) with tartaric acid, add 0.03% (g / ml) needles, stir well with activated carbon, stir and absorb for 20 minutes, decarbonize an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com