Pharmaceutical composition of ondansetron hydrochloride and dexamethasone
A technology of ondansetron hydrochloride and ondansetron hydrochloride, applied in the field of medicine, can solve problems such as unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
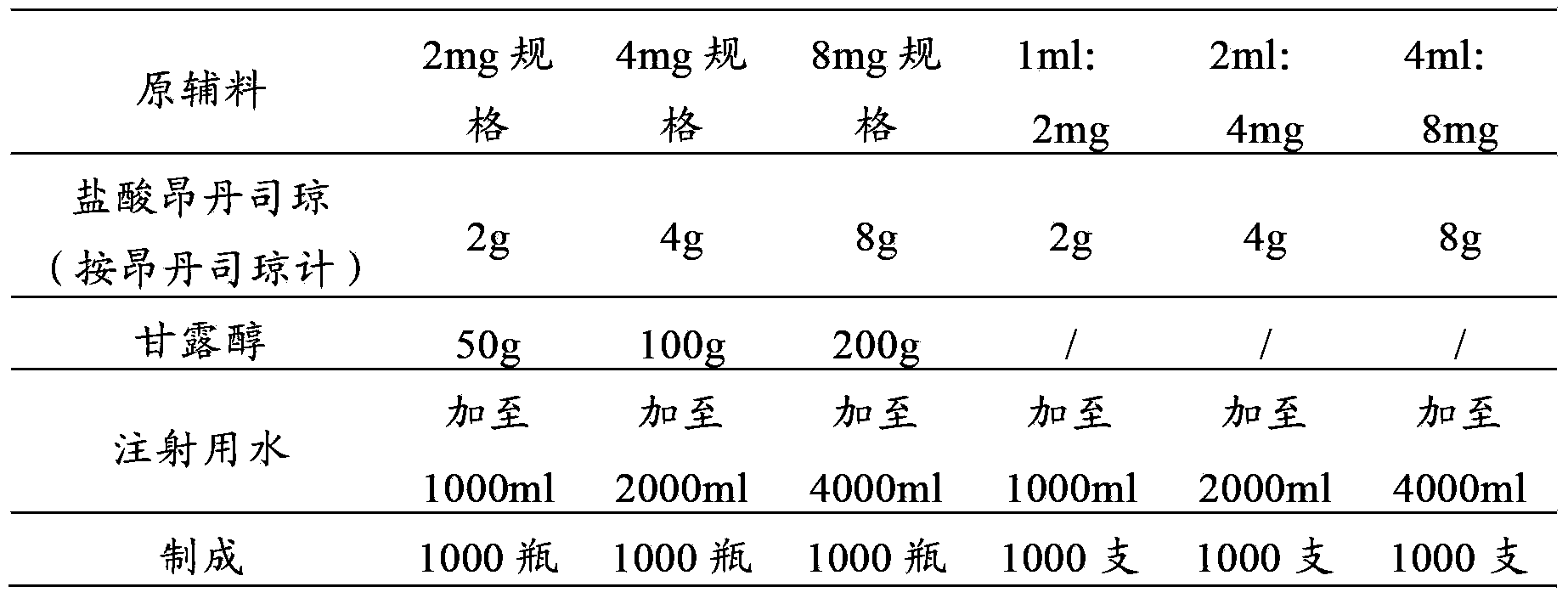
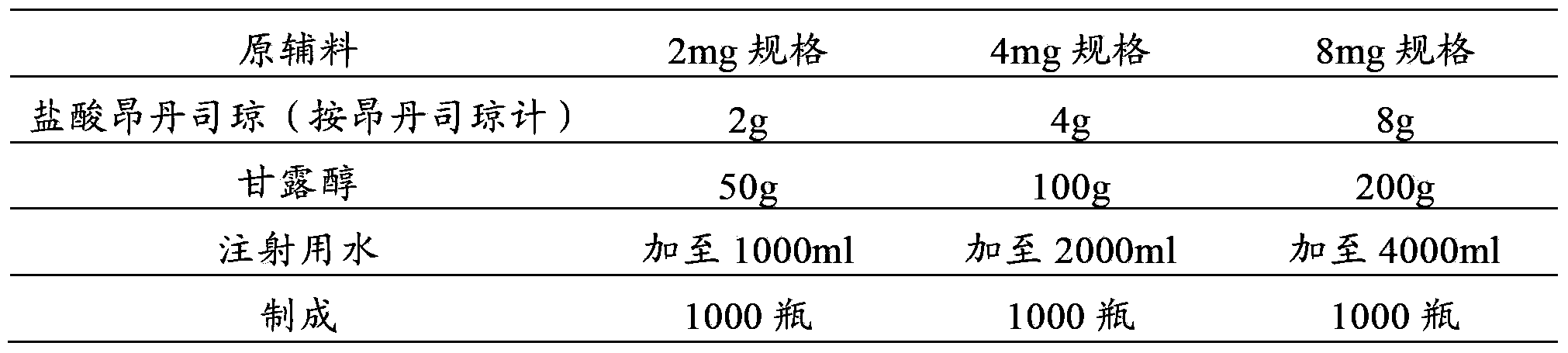
[0035] Example 1 Preparation of Ondansetron Hydrochloride Freeze-dried Powder for Injection
[0036] prescription:
[0037]
[0038] Preparation Process:
[0039] (1) First add 5600ml water for injection into the container;
[0040] (2) Add 14g of ondansetron hydrochloride (calculated as ondansetron) and 350g of mannitol, stir to dissolve completely, and adjust the pH to 3.6 with 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution;
[0041] (4) Add 1.4g of activated carbon for injection, add the remaining water for injection, set the volume to 7000ml, stir and absorb for 30 minutes;
[0042] (5) The solution is decarbonized by coarse filtration, coarsely filtered through a 0.45 μm cartridge filter, and then sterilized and filtered with a 0.22 μm microporous membrane until the visible foreign matter is qualified;
[0043] (6) Filling, 1ml / bottle or 2ml / bottle or 4ml / bottle.
[0044] (7) Freeze drying
[0045] ① During the pre-freezing period, lower th...
Embodiment 2
[0049] Example 2 Preparation of Ondansetron Hydrochloride Injection
[0050] prescription:
[0051]
[0052] Preparation Process:
[0053] (1) First add 5600ml water for injection into the container;
[0054] (2) Add 14 g of ondansetron hydrochloride (calculated as ondansetron), stir to dissolve completely, and adjust the pH to 3.5 with 1mol / L sodium hydroxide solution or 1mol / L hydrochloric acid solution;
[0055] (4) Add 1.4g of activated carbon for injection, add the remaining water for injection, set the volume to 7000ml, stir and absorb for 30 minutes;
[0056] (5) The solution is decarbonized by coarse filtration, coarsely filtered through a 0.45 μm cartridge filter, and then sterilized and filtered with a 0.22 μm microporous membrane until the visible foreign matter is qualified;
[0057] (6) Filling, 1ml / bottle or 2ml / bottle or 4ml / bottle, seal and sterilize.
Embodiment 3
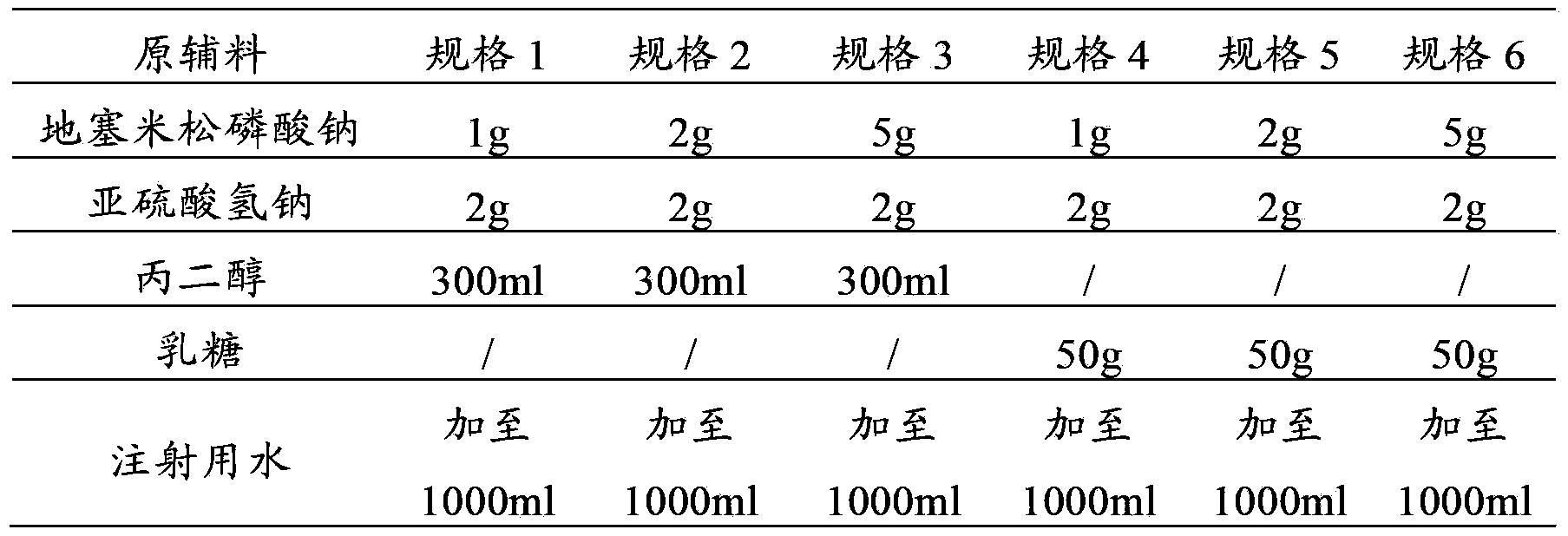
[0058] Example 3 Preparation of Dexamethasone Sodium Phosphate Injection
[0059] prescription:
[0060]
[0061] Preparation Process:
[0062] (1) Add 500ml of water for injection into the beaker, add 2g of sodium bisulfite and 300ml of propylene glycol, stir to dissolve, then add 1g of dexamethasone sodium phosphate, stir to completely dissolve, use 0.1mol / L hydrochloric acid solution or 0.1 mol / L sodium hydroxide solution to adjust the pH to 7.8, then add 0.5g of wetted medicinal charcoal, add water for injection to 1000ml, stir for 30 minutes, filter and decarbonize; fine filter with a 0.22μm microporous membrane, Filling, 1ml / bottle, sealing; autoclave at 121°C for 15 minutes, quickly spray to cool down, take out the cabinet, and naturally cool to room temperature.
[0063] (2) Add 500ml of water for injection into the beaker, add 2g of sodium bisulfite and 300ml of propylene glycol, stir to dissolve, then add 2g of dexamethasone sodium phosphate, stir to completel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com