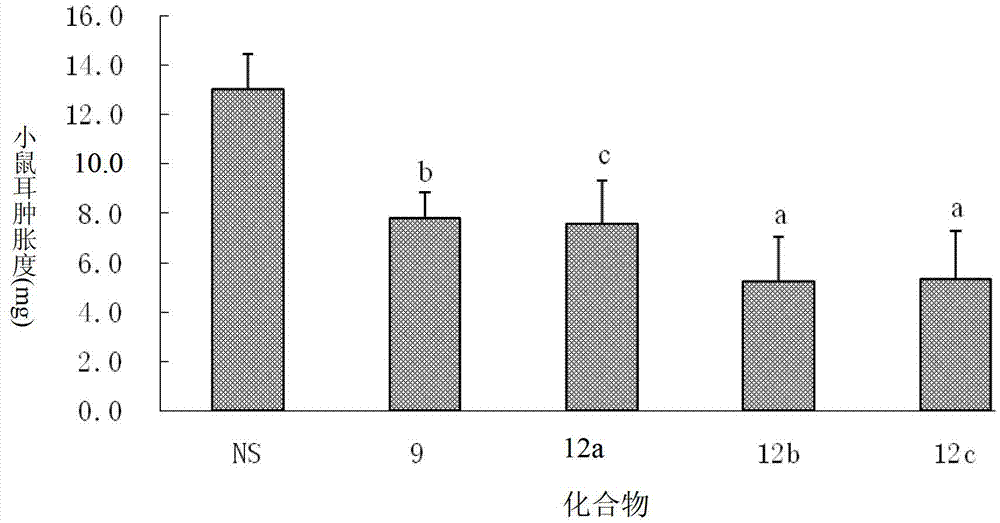
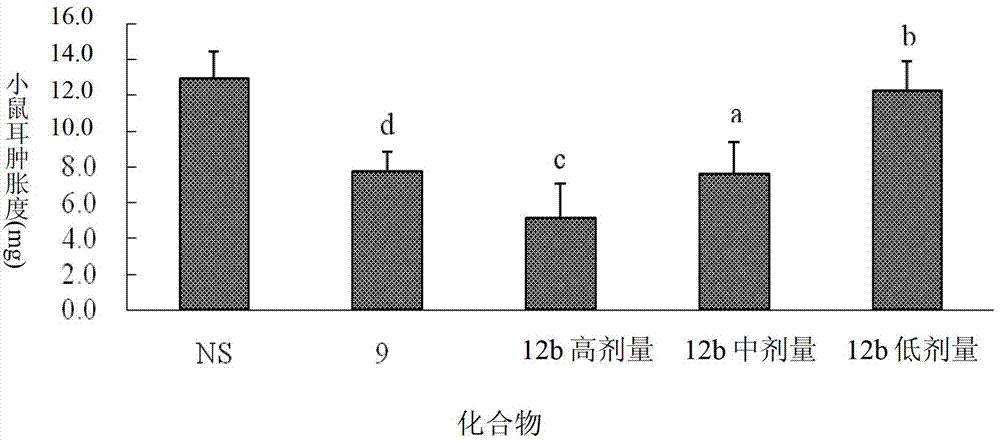
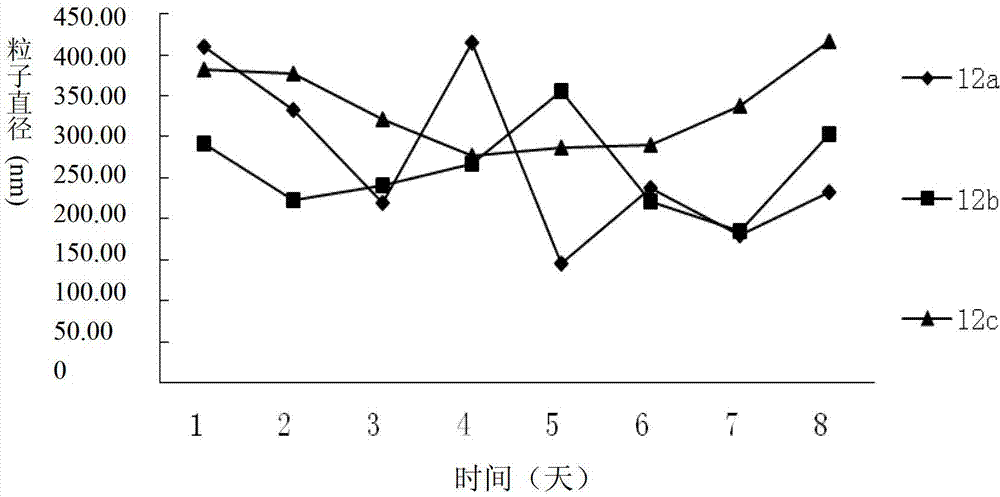
Dexamethasone-RGD polypeptide conjugate, preparation method thereof and application
A technology of dexamethasone and conjugates, applied in the field of dexamethasone-RGD polypeptide conjugates, can solve problems such as osteoporosis, and achieve the advantages of reduced side effects of osteoporosis, excellent immunosuppressive effect, and excellent anti-inflammatory effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 prepares BocArg (NO 2 )-Gly-OBzl
[0036] 800mg (2.5mmol) Boc-Arg (NO 2 )-OH was dissolved in an appropriate amount of anhydrous THF, and anhydrous THF solution of 338 mg (2.5 mmol) HOBt and 619 mg (3.0 mmol) DCC was added under ice cooling. After 20 minutes of reaction, the corresponding active ester was obtained, which was directly used in the following reaction.
[0037] 775mg (2.3mmol) Tos·Gly-OBzl and 230mg (2.3mmol) N-methylmorpholine were miscible with an appropriate amount of anhydrous THF, added to the above reaction solution, and reacted at room temperature for 24 hours. TLC (CH 2 Cl 2 :MeOH=20:1) shows that the raw material point disappears. Spin to dry the solvent, add 150ml ethyl acetate, wash with saturated NaHCO 3 30ml×3, saturated sodium chloride aqueous solution 30ml×3, saturated KHSO 4 30ml×3, after washing with saturated aqueous sodium chloride solution 30ml×3, the ethyl acetate layer was washed with anhydrous NaCl2 SO 4 Dry and ...
Embodiment 2
[0038] Embodiment 2 prepares Boc-Arg (NO 2 )-Gly
[0039] 1.167g (2.5mmol) Boc-Arg (NO 2 )-Gly-OBzl dissolved in an appropriate amount of anhydrous CH 3 OH, add 2.5ml (5mmol) 2N NaOH aqueous solution under ice-cooling. Reacted for 30 minutes, TLC (CH 2 Cl 2 :MeOH=20:1) shows that the raw material point disappears, add saturated KHSO 4 Adjust pH-7, spin dry methanol, add saturated KHSO 4 Adjust pH-2, extract with ethyl acetate, wash the ethyl acetate layer with anhydrous Na 2 SO 4 Dried, filtered and spin-dried to obtain 1.075g, the yield (90%) was directly used in the following reaction.
Embodiment 3
[0040] Embodiment 3 prepares Boc-Ser(Bzl)-OBzl
[0041] 739mg (2.5mmol) Boc-Ser(Bzl)-OH was dissolved in 10ml absolute ethanol, and 405mg (1.25mmol) Cs was added 2 CO 3 The aqueous solution was reacted at room temperature for 15 minutes, and TLC (ethyl acetate:petroleum ether=1:1) showed that the starting point disappeared. Concentrate under reduced pressure to remove the solvent to obtain the corresponding cesium salt Boc-Ser(Bzl)OCs. The obtained cesium salt was first dissolved in 2 ml of anhydrous DMF, and then 0.3 ml (2.5 mmol) of benzyl bromide was added thereto. The reaction mixture was stirred at 55-60 ° C for 4 h, TLC (CHCl 3 :MeOH=30:1) shows that the cesium salt of Boc-Ser(Bzl) disappears. The reaction mixture was concentrated to dryness under reduced pressure, the residue was dissolved in ethyl acetate, and the insoluble matter was filtered off. The filtrate was successively washed with saturated NaHCO 3 30ml×3 and 30ml×3 saturated aqueous sodium chloride solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com