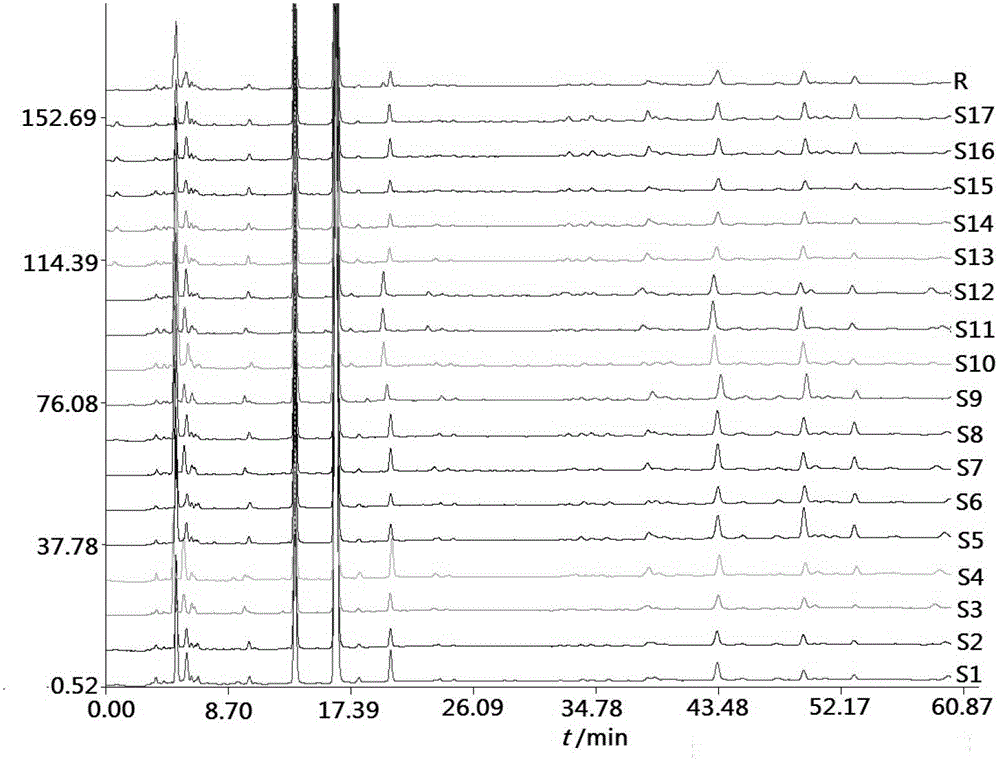
Fingerprint building method and quality control method of Shenxiong glucose injection raw material, Shenxiong glucose injection midbody and Shenxiong glucose injection preparation
A technology of ginseng glucose and fingerprint spectrum, which is applied in the field of establishment method and quality control of traditional Chinese medicine fingerprint spectrum, and can solve problems such as difficulty in comprehensively reflecting the characteristics of chemical components of preparations.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] Two, the preparation of reference substance solution
[0061] Accurately weigh the appropriate amount of danshensu sodium, ligustrazine hydrochloride, protocatechualdehyde, salvianolic acid D, rosmarinic acid, salvianolic acid B, and salvianolic acid A, add methanol to dissolve, and dilute to make the concentrations 1mg·mL respectively -1 of the reference substance solution.
[0062] Three, the preparation of test solution
[0063] 1. Preparation of Salvia miltiorrhiza medicinal material test solution
[0064] Take about 0.5g of Salvia miltiorrhiza powder (raw material) through a 40-mesh sieve, weigh it accurately, add 25ml of water precisely, weigh it, heat and reflux for extraction for 2 hours, let it cool and weigh it again, make up the lost weight with water, shake well, The supernatant was filtered with a microporous membrane (0.45 μm), and the filtrate was taken to obtain the test solution of Danshen medicinal material.
[0065] 2. Preparation of Salvia miltior...
Embodiment 1
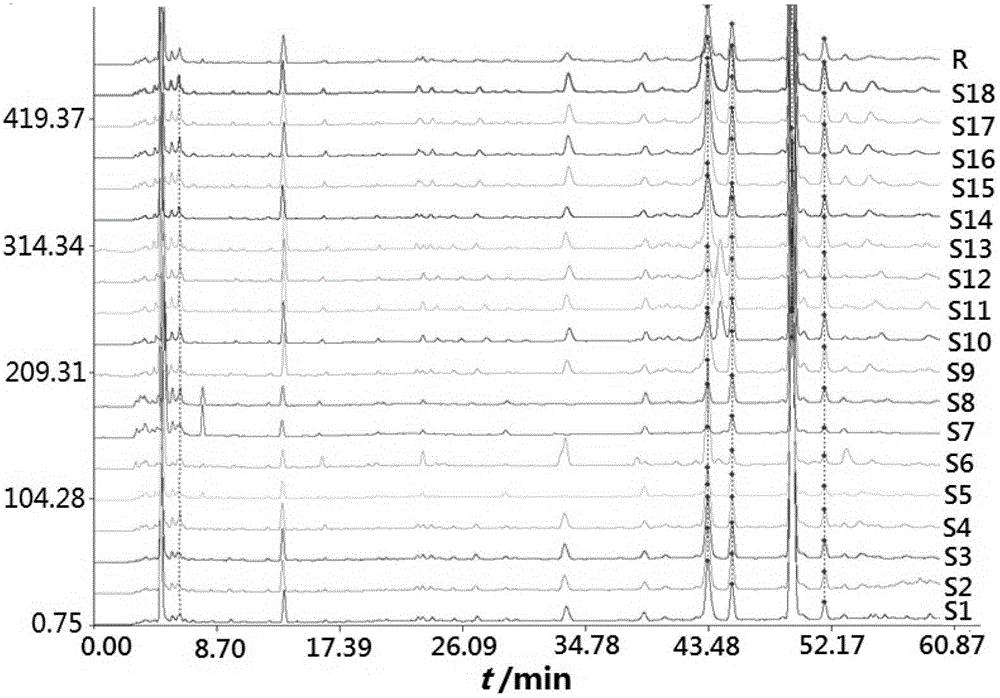
[0083] Embodiment 1: Determination of Fingerprint Spectrum of Salvia Miltiorrhiza
[0084] (1) Source of medicinal materials
[0085] The samples of Salvia miltiorrhiza came from Sichuan, Shandong, Henan, Shaanxi and Jiangsu, etc. The details are shown in Table 1, and were identified by the Department of Medicinal Plants and Pharmacognosy of Guiyang Medical College.
[0086] Table 118 batches of Danshen medicinal materials source and origin
[0087]
[0088] (2) Chromatographic conditions
[0089] DiamonsilC18 column (4.6mm×250mm, 5μm); mobile phase acetonitrile (A)-0.05% phosphoric acid (B), flow rate 1mL min -1 , gradient elution, 0~7min, A2%~4%; 7~12min, A4%~18%; 12~15min, A18%~20%; 15~22min, A20%~23%; 22~32min, A23 %~32%; 32~42min, A32%~35%; 42~50min, A35%~90%; 50~60min, A90%. The column temperature is 40°C, the detection wavelength is 230nm, the injection volume is 10μL, and the chromatogram is recorded for 60min.
[0090] (3) Investigation on the preparation meth...
Embodiment 2
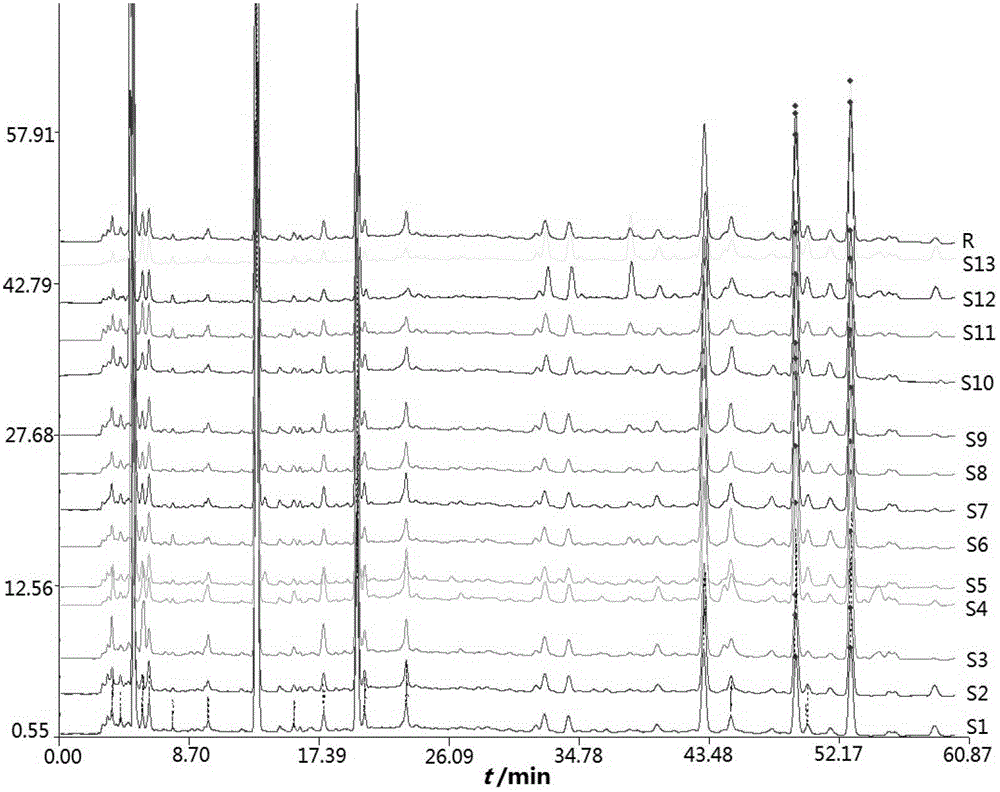
[0107] Embodiment 2: The fingerprint chromatogram of semi-finished product of Danshen root is measured
[0108] (1) Chromatographic conditions
[0109] DiamonsilC18 column (4.6mm×250mm, 5μm); mobile phase acetonitrile (A)-0.05% phosphoric acid (B), flow rate 1mL min -1 , gradient elution, 0~7min, A2%~4%; 7~12min, A4%~18%; 12~15min, A18%~20%; 15~22min, A20%~23%; 22~32min, A23 %~32%; 32~42min, A32%~35%; 42~50min, A35%~90%; 50~60min, A90%. The column temperature is 40°C, the detection wavelength is 230nm, the injection volume is 10μL, and the chromatogram is recorded for 60min.
[0110] (2) Preparation of the test solution
[0111] Take about 0.15g of the semi-finished product of Salvia miltiorrhiza, weigh it accurately, dissolve and dilute it with water, set the volume to 50mL, shake well, filter the supernatant with a microporous membrane (0.45μm), and take the subsequent filtrate to obtain the test solution of the semi-finished product of Salvia miltiorrhiza ;
[0112] (3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com