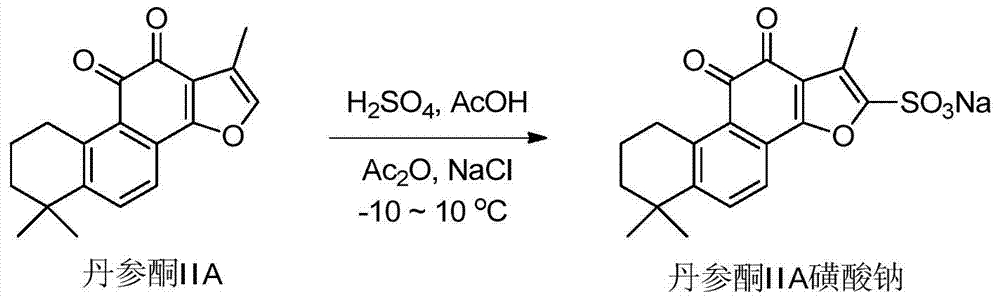
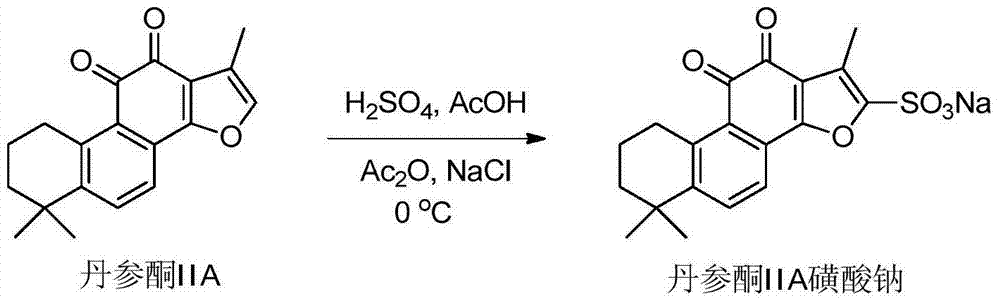
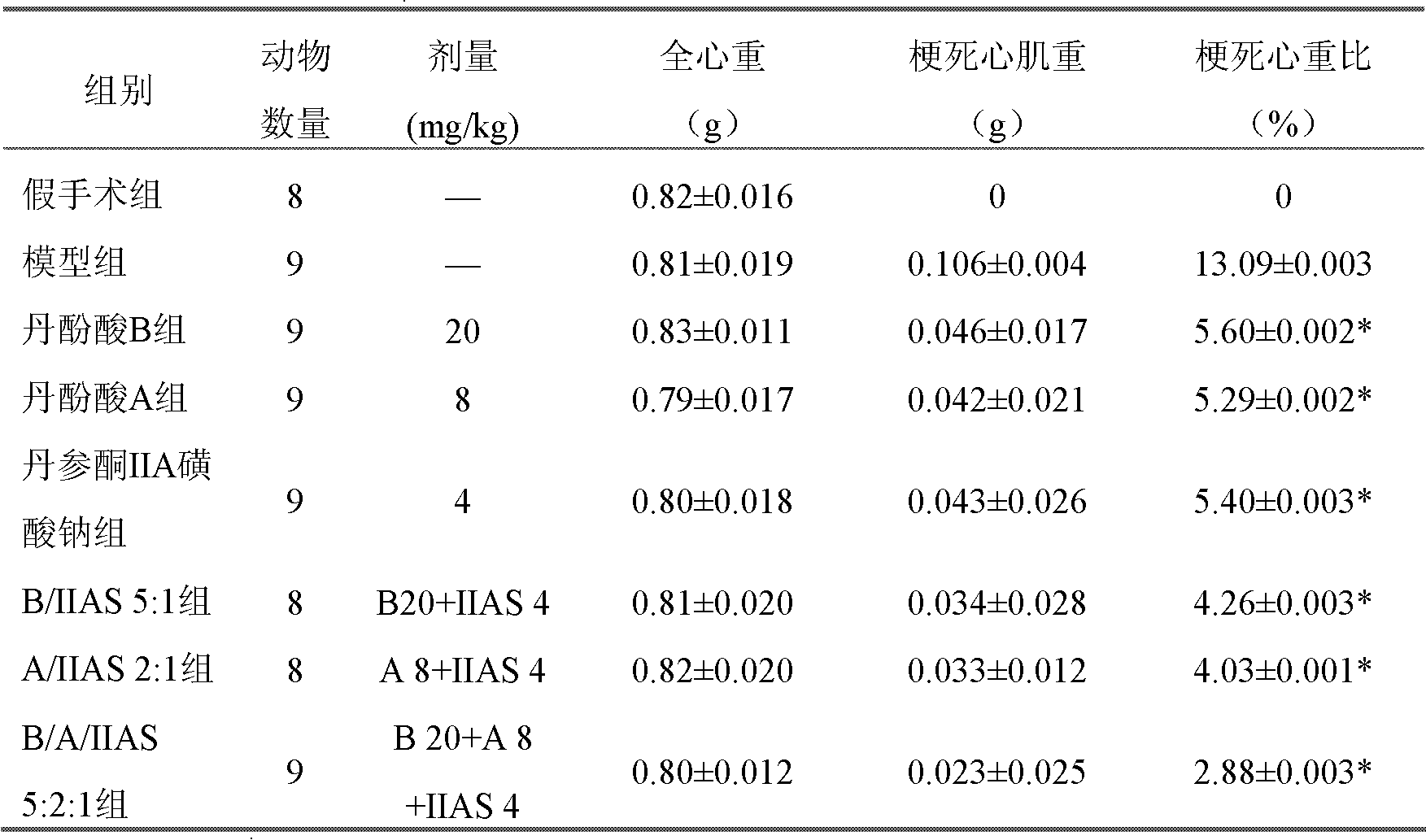
Patents
Literature
66 results about "Sodium tanshinone IIA sulfonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
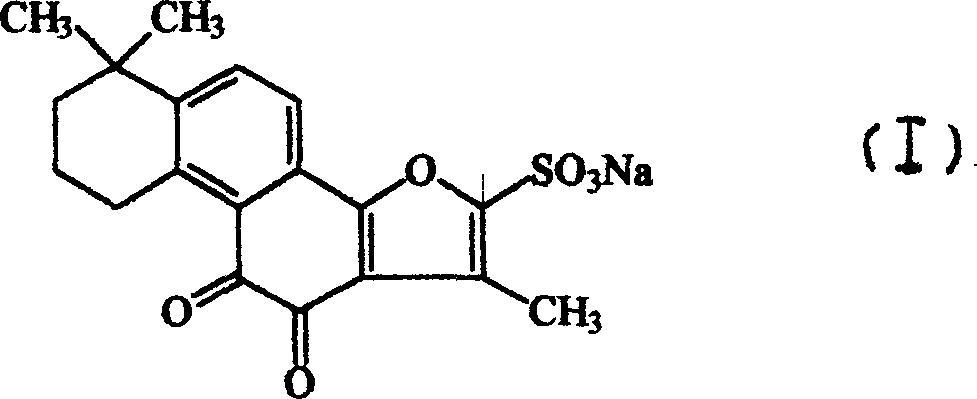
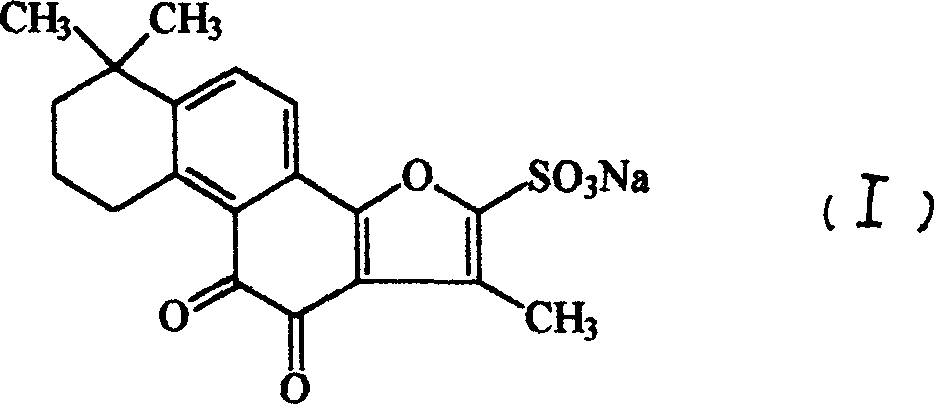
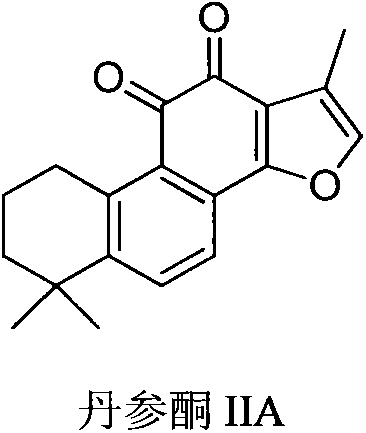
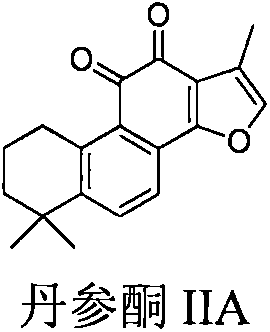
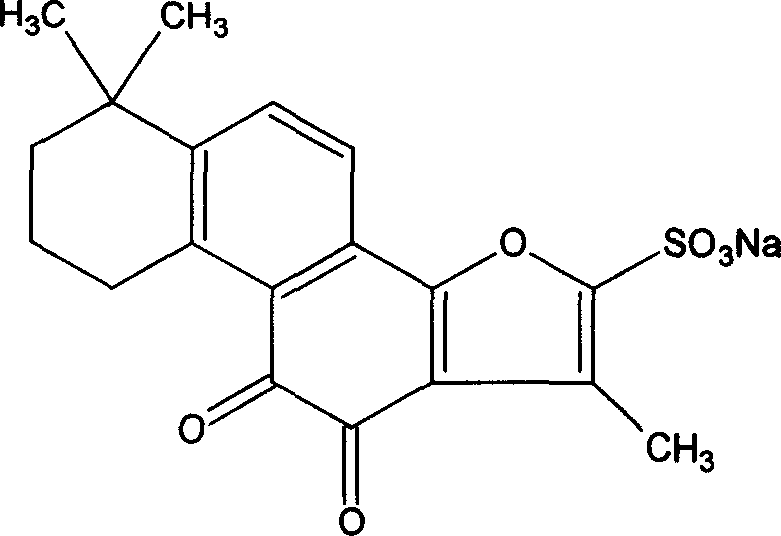
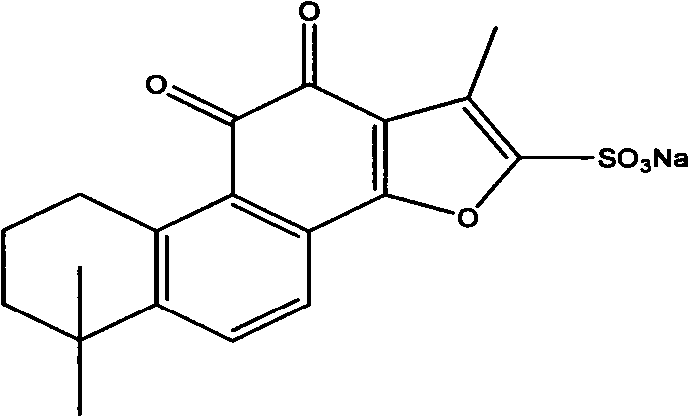
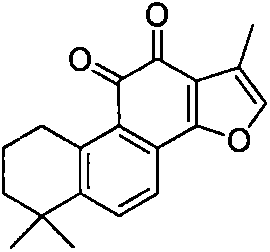
Sodium tanshinone IIA sulfonate (STS) is a water-soluble derivative of tanshinone IIA isolated as the main pharmacologically active natural compound from a traditional Chinese herbal medicine, the dried root of Salvia miltiorrhiza Bunge known as Danshen.
Tanshinon IIA sulfonic acid sodium glucose injection liquid and its preparing method
InactiveCN1640391ASolve discolorationFix instabilityOrganic active ingredientsPharmaceutical delivery mechanismAntioxidantTherapeutic effect
The persent invention relates to a sodium tanshinon IIA sulfonate glucose injection and its preparation method. Said injection contains (by wt%) 0.002-0.016% of sodium tanshinon IIA sulfonate, 5-25% of glucose, 0.05-0.2% of antioxidant and the rest is injection water. Said injection can obtain good therapeutic effect, and its preparation method is simple.
Owner:昆明希捷医药研发有限公司
Method for separating and purifying impurities in sodium tanshinone IIA sulfonate crude drug
InactiveCN102936275AOvercome operabilityOvercome the cycleSteroids preparationSulfonateDehydrogenation
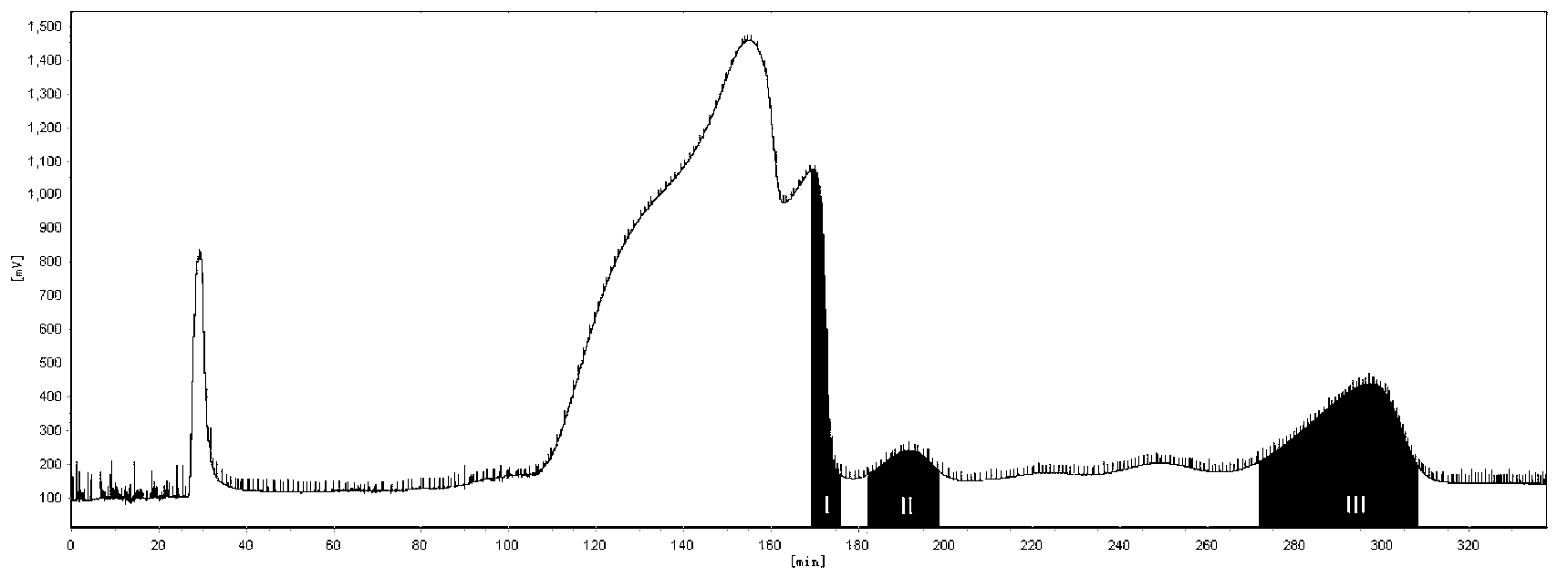
The invention relates to the technical field of medicines and discloses a method for separating and purifying impurities in a sodium tanshinone IIA sulfonate crude drug. Sodium tanshinone IIA sulfonate is widely used for treating cardiovascular and cerebrovascular diseases and other systemic disease at present. 1, 2-dehydrogenation sodium tanshinone IIA sulfonate, sodium tanshinone IIB sulfonate and 1-hydroxy sodium tanshinone IIA sulfonate are three major impurities in the sodium tanshinone IIA sulfonate crude drug. The method for separating and purifying the impurities in the sodium tanshinone IIA sulfonate crude drug is that a high-speed counter-current chromatography method is used for separating and purifying the three major impurities in the sodium tanshinone IIA sulfonate crude drug. The method has the advantages of being simple, fast, high in recovery rate, large in preparation quantity, high in separation efficiency, good in product purity, convenient and suitable for studying comparison products, and defects of cumbersome operations and long separation periods of traditional preparation methods are overcome.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for refining tanshinoneIIA sodium sulfonate
InactiveCN101200489AHigh purityComply with the requirements of raw materials for injectionSteroids preparationSulfonateOrganic solvent
The invention relates to a refining method of tanshinone II sulfonate. The method comprises the following procedures, firstly the crude tanshinone II sulfonate to be purified is added into the mixture solution of the organic solvent and water, heated to 40 to 60 DEG C, agitated to dissolve and filtered to remove the insoluble particles; secondly, the filtrate is slowly decreased to the temperature of 0 to 30 DEG C and crystallized and filtered; thirdly tanshinone II sulfonate with high purity is obtained after the vacuum drying. The contents of tanshinone II sulfonate can reach 99.5 percent and the quality can meet the purity requirement of raw medicine for injection by HPLC analysis. The method of the invention has the advantages of simple method, easy operation, low refining cost and being suitable for the industrialized production.
Owner:YAOPHARMA CO LTD
Tanshinone II A sodium sulfonate powder injection agent and its preparation method
InactiveCN1555791AImprove qualityImprove stabilityPowder deliveryOrganic active ingredientsVitamin CAntioxidant
A tanshinone IIAsodium sulfonate injection in the form of powder is prepared from tanshinone IIA sodium sulfonate (50-100 wt.%) and medical auxiliaries (0-50 wt.%) including skeleton supporting agent and antioxidizing agent.
Owner:昆明同持医药研究有限公司
High purified tanshinone óÄA sodium sulfonate, fabricating method, and preparation
InactiveCN1915986AQuality improvementOrganic active ingredientsPowder deliverySulfonateFreeze-drying
This invention relates to a method for preparing high-purity sodium tanshionone IIA sulfonate, and preparations containing the compound. Determined by HPLC, the content of sodium tanshionone IIA sulfonate is higher than 96%, the total content of the related materials is lower than 10%, and the content of individual impurities is lower than 4.0%. The injection of high-purity sodium tanshionone IIA sulfonate can avoid the problems of flotation and precipitation of components. Besides, the compounds can also be manufactured into large-volume infusion and freeze-dried powder injection.
Owner:JILIN SIHUAN PHARM CO LTD
Synthesis and application of sulfonamide compounds
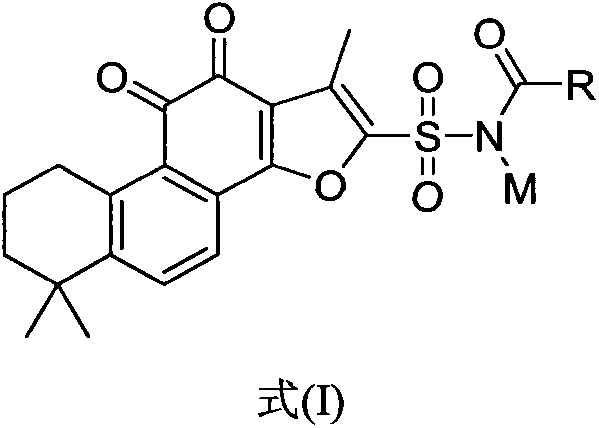
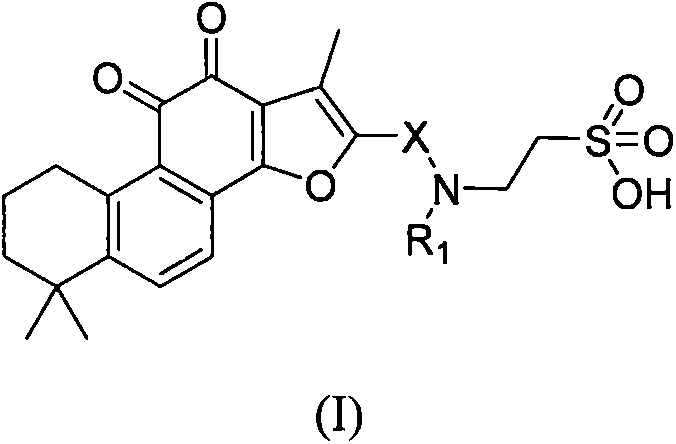
The invention relates to tanshinone IIA derivatives. The structure of the tanshinone IIA derivatives are carbonylated and acylated products of tanshinone IIA furan ring 2-position sulfonamide as show in a formula (I), wherein R is C1-6 alkyl, cycloalkyl, alkenyl, alkynyl, aryl and aromatic heterocycle and M is a metal ion such as a sodium salt. According to the compounds, on the basis that the activity and water solubility of sodium tanshinone IIA sulfonate are maintained, the acidity of the compounds is reduced; when sodium tanshinone IIA sulfonate is injected, the stimulation to patients is reduced and the stability of the compounds is improved.
Owner:北京桦冠医药科技有限公司
Method of preparing high-purity tanshinone IIA sodium sulfonate
The invention provides a method of preparing high-purity tanshinone IIA sodium sulfonate. According to the method, a tanshinone IIA crude product is subjected to a sulfonation reaction, side reactions are reduced by means of control over the reaction temperature, and an organic solvent extraction method is used for removing organic soluble impurities which are not reacted so as to obtain the high-purity tanshinone IIA sodium sulfonate. By means of high performance liquid chromatography for measurement, the content of tanshinone IIA sodium sulfonate in the product is above 95%, and the quantity of a single impurity is below 2%.
Owner:KUNMING INST OF BOTANY - CHINESE ACAD OF SCI
Tanshinone II A sodium sulfonate and method of determining tanshinone I sodium sulfonate in its preparation
ActiveCN1624474AAccurate measurementEasy to measureComponent separationChromatographic separationSilanes
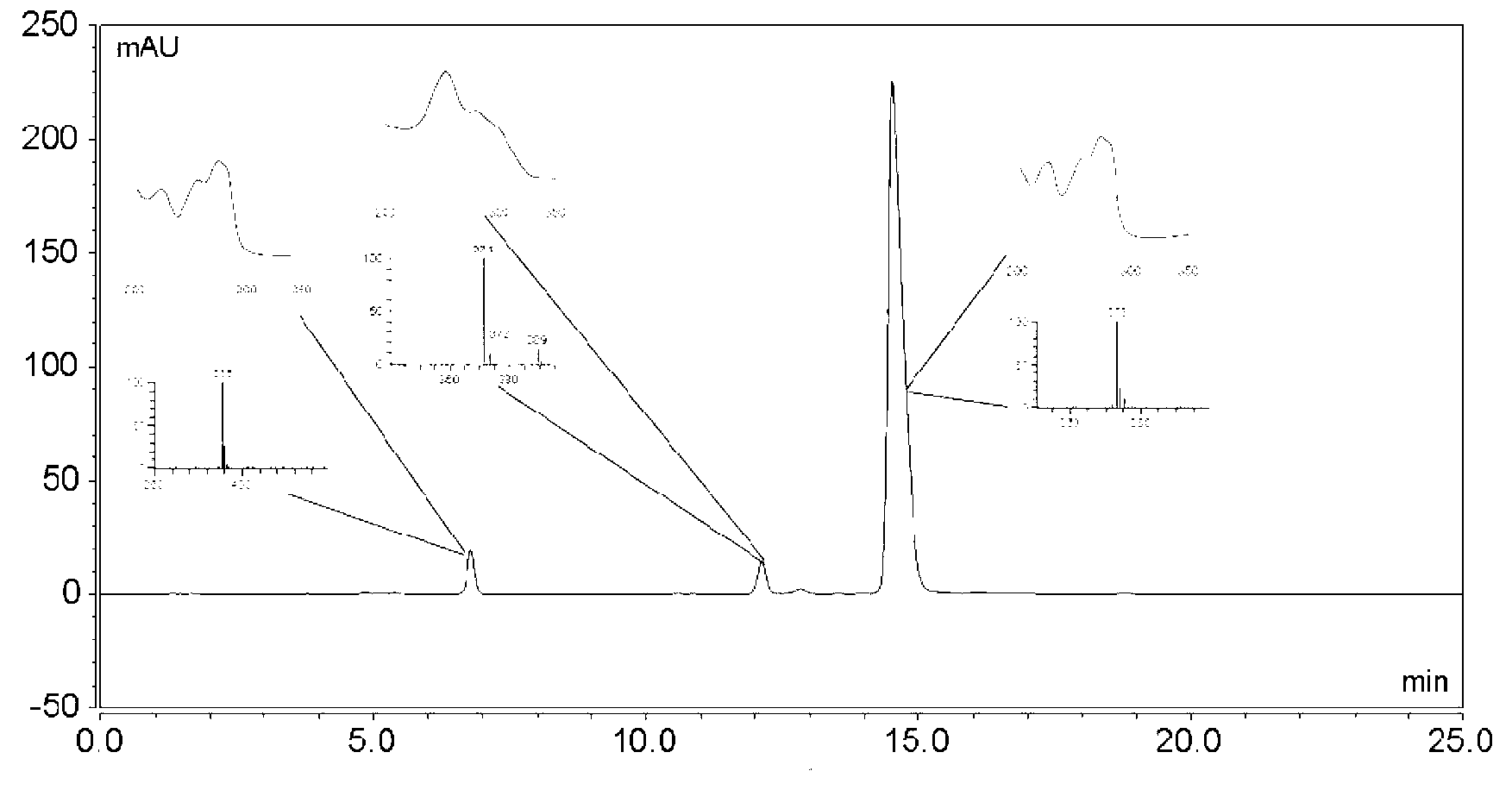
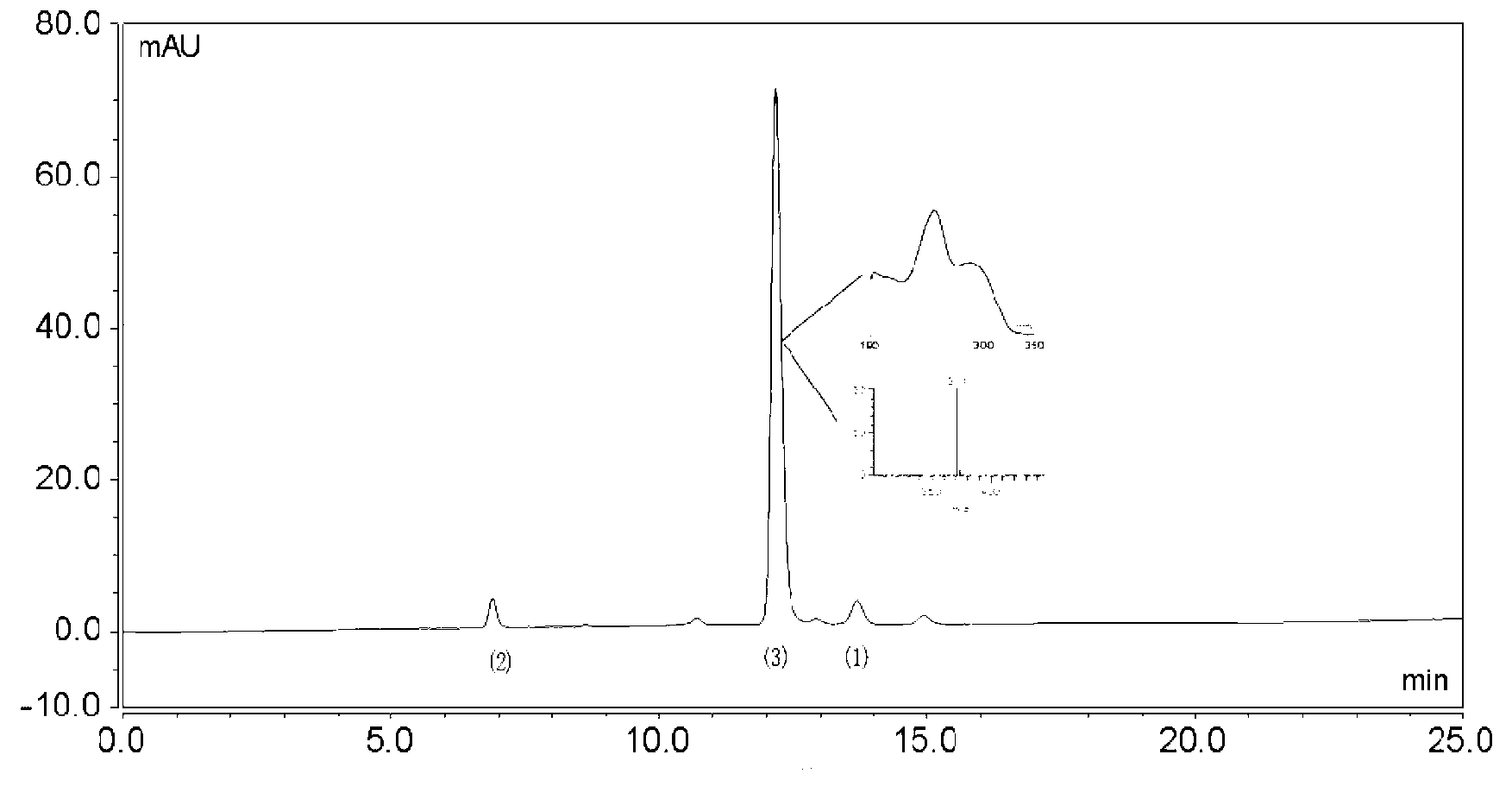
The invention relates to a qualitative and quantitative measuring method for tanshinone IIA sodium mercuric thiocyana and tanshinone I sodium mercuric thiocyana in its preparation, including the following steps: 1) use highly efficient liquid phase chromatography method, choose at least one as filling material from octadecyl silane linking aerosol, and aerosol linking aerosol,; make methyl alcohol- -buffer solution as mobile phase 6 in which the volume rate of methyl alcohol is 9-90%, 8 is 0-90%, buffer solution is 5-90% and the PH value of it is 2-5; 2) inject the sample into the highly efficient liquid phase chromatograph to do chromatographic fractionation and ultraviolet test. The advantages are high accuracy, simple measurement and high reliability.
Owner:SHANDONG BESTCOMM PHARMA CO LTD
Danshinone II A sodium sulphonate freeze dried powder injection agent and its preparation method
InactiveCN1686105AImprove stabilityStable contentOrganic active ingredientsPowder deliveryDiseaseFreeze-drying
A freeze-dried powder injection of danshinone IIA-sodium sulfonate for treating coronary heart disease, angina pectoris, palpitation, chest distress, etc and its preparing process are disclosed.
Owner:刘歆
Tanshinone óÄA sodium sulfonate for injection and its preparation method
InactiveCN1634024AAccurate therapeuticAccurate rescuePowder deliveryOrganic active ingredientsSulfonateTanshinone IIA
The invention relates to a dosage type of tanshinone III sodium sulfonate, and the process for preparing the dosage type, which comprises tanshinone IIA sodium sulfonate and framework material, and the weight ratio of the tanshinone IIA sodium sulfonate and framework material is, tanshinone IIA sodium sulfonate 10-70% and framework material 30-90%. The invention has the advantages of accurate dosage, good stability, thus can be applied to the treatment and rescue for patients.
Owner:昆明希捷医药研发有限公司
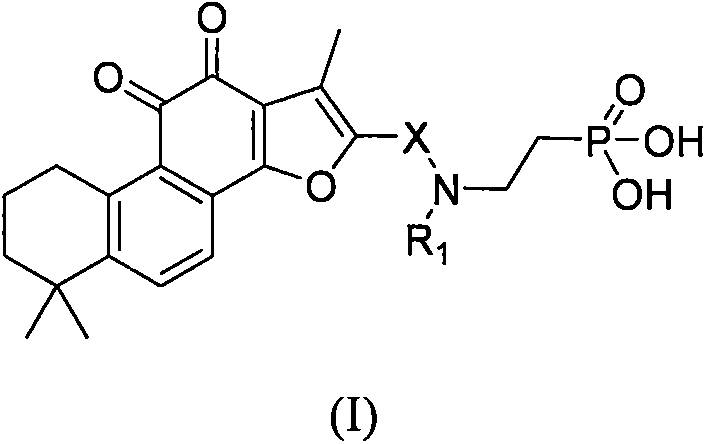
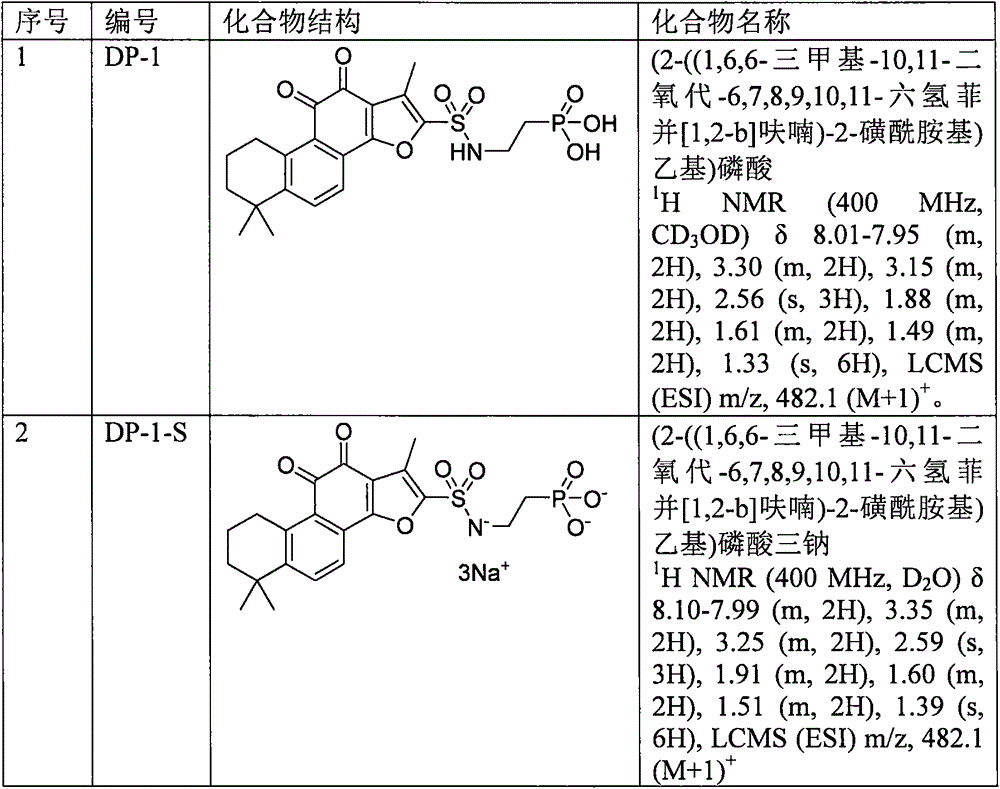
Tanshinone IIA phosphoric acid derivative and synthesis and use thereof as medicine
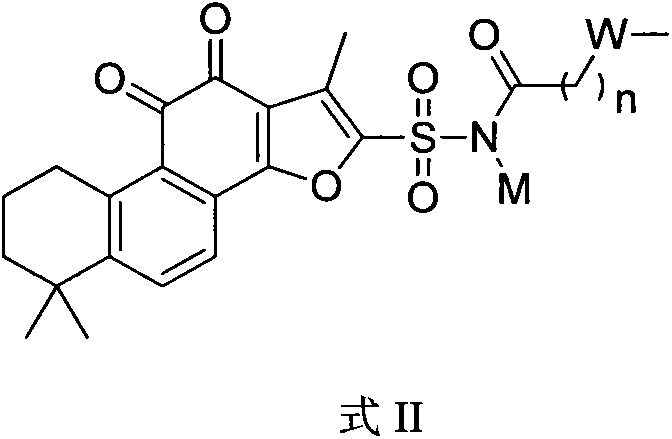
The invention discloses a tanshinone IIA phosphoric acid derivative and synthesis and use thereof as a medicine for treatment. In particular, the present invention provides a novel derivative shown as a general formula (I) and a pharmaceutically acceptable salt thereof or a pharmaceutical composition containing the novel derivative shown as the general formula (I), and a method for preparing the novel derivative shown as the general formula (I). The invention also discloses use of the novel derivative, the pharmaceutically acceptable salt thereof or the pharmaceutical composition containing the novel derivative in preparation of medicines for treatment of coronary heart disease, angina, myocardial infarction, viral myocarditis, arrhythmia, cerebrovascular disease, hepatitis, pulmonary heart disease, bronchial asthma, cancer, kidney disease, eye disease, thromboangiitis obliterans, hypertension, fractures, burns, surgical operations or behcet ' s syndrome. On the basis of ensurance of the activity of tanshinone IIA, the novel derivative, the water solubility of the pharmaceutically acceptable salt thereof or the pharmaceutical composition containing the novel derivative can be improved. Compared with tanshinone IIA sodium sulfonate, the stability is improved, and because the acidity of the pharmaceutically acceptable salt thereof or the pharmaceutical composition containing the novel derivative is reduced obviously, injection stimulation can be avoided. All substituents of the general formula (I) are as defined in the specification.
Owner:北京益佰医药研究有限公司
Method for preparing tanshinone IIA sodium sulfonate by using tanshinone crude extract
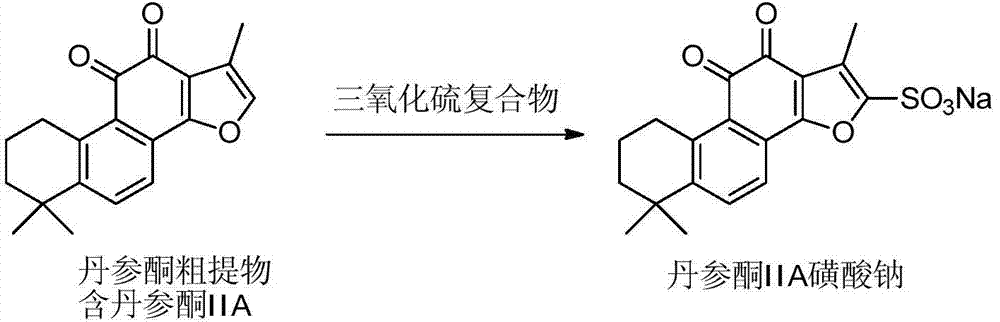
The invention provides a method for preparing tanshinone IIA sodium sulfonate by directly adopting a tanshinone crude extract. According to the method, the tanshinone crude extract is directly subjected to sulfonation reaction in an organic solvent by using a sulfur trioxide or sulfur trioxide complex, after the reaction is ended, a reaction solution is filtrated, washing is carried out by using an organic solvent so as to remove unreacted impurities in the tanshinone crude extract and other impurities which can be dissolved in the organic solvent, and the obtained filter cake is dissolved and then is converted into tanshinone IIA sodium sulfonate by using a sodium containing reagent. The method has the advantages that the sulfonation reaction is carried out by adopting an environmental-friendly reagent instead of chemical reagents, such as concentrated sulfuric acid, acetic acid, acetic anhydride and the like, which cause serious environmental pollution. Meanwhile, the reaction is carried out by directly adopting the tanshinone crude extract as a raw material, so that the cost caused by adopting high-purity tanshinone IIA as the raw material is reduced, byproducts of the obtained product are few, and the yield and purity of the product are high.
Owner:华药控股有限公司
Application of tanshinone IIA sodium sulfonate as blood-brain barrier protecting agent in cerebral diseases
InactiveCN101120940AExact therapeutic effectExact preventive effectOrganic active ingredientsNervous disorderDiseaseTanshinone IIA
The present invention belongs to pharmaceutical field, which relates to the application of the sodium tanshinone IIA sulfonate as a protective agent for the blood brain barrier in mental diseases. The present invention provides the new function of the sodium tanshinone IIA sulfonate as a protective agent for the blood brain barrier in mental disease prevention and treatment. The in vitro and in vivo studies display that the sodium tanshinone IIA sulfonate possesses definite protection function for the blood brain barrier. The present invention also possesses definite prevention and treatment function for metal disease related to the blood brain barrier injury, which comprises ischemic cerebral apoplexy. The present invention skillfully takes the blood brain barrier as the therapy target for mental diseases, which breaks through the restriction that the treatment medicine should reach the effective drug therapy concentration in accordance with the pharmacokinetic characteristics. Medicine provided in the present invention can protect the brain when the drug reaches effective intravascular concentration. Therefore better choice for treatment of the mental disease is provided.
Owner:CHINA PHARM UNIV
Hydroxy tanshinone IIA sodium sulfonate and its application
ActiveCN1844113AEffective monitoringEnsure safetyPowder deliveryOrganic active ingredientsSulfonateTanshinone IIA
The invention discloses a hydroxyl sodium tanshinone IIA silate having a molecular structural formula disclosed in the specification, and the use of the compound as foreign substance for comparison in inspecting sodium tanshinone IIA silate raw material and sodium tanshinone IIA silate preparations in the forms of sodium tanshinone IIA silate liquid injection, sodium tanshinone IIA silate freeze dried injection, sodium tanshinone IIA silate large volume injection, and sodium tanshinone IIA silate oral solid preparation.
Owner:JIANGSU CAREFREE PHARM CO LTD
Medicinal composition of radix salviae miltiorrhizae extract and application thereof
InactiveCN102552398AOrganic active ingredientsCardiovascular disorderSalvianolic acid BSalvia extract
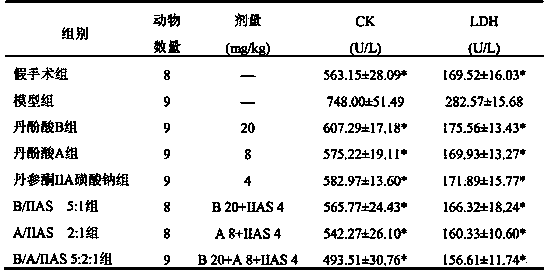
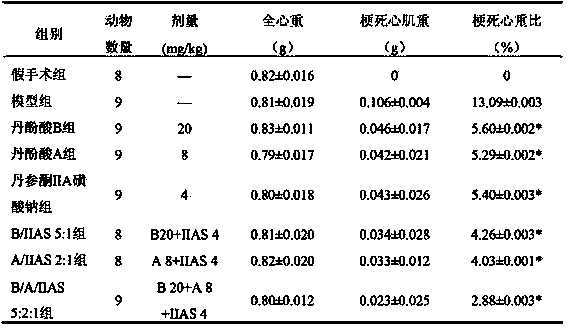
The invention relates to a medicinal composition of radix salviae miltiorrhizae extract and application thereof. The medicinal composition is the combination of salvianolic acid A or salvianolic acid B and tanshinone IIA sodium sulfonate, or combination of salvianolic acid A and salvianolic acid B and tanshinone IIA or tanshinone IIA sodium sulfonate. The therapeutic effect of the medicinal composition on myocardial infarction is better than that when the components are independently used; the multi-component multi-target therapeutic effect of the traditional Chinese medicine radix salviae miltiorrhizae can be better reflected; and the medicinal composition is used for preparing a medicine for treating vascular diseases. The medicinal composition can be processed into tablets, capsules, granules, freeze-dried preparation for injection, or freeze-dried preparation for injection or powder injection of carboxylate thereof.
Owner:SHANDONG UNIV
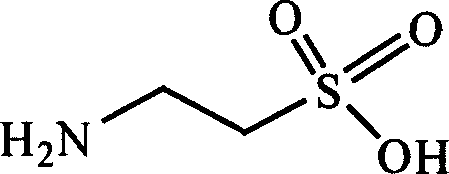
Taurine and medical combination for treating cardiovascular and cerebrovascular diseases
InactiveCN101062027AMeet urgent clinical needsImprove pharmacological activityOrganic active ingredientsSuppositories deliveryDiseaseCoronary heart disease
The invention discloses a medical component with taurine and medicine to treat blood vessel of brain and heart disease, preparing method and usage in medical technical domain, which is characterized by the following: choosing the blood vessel of brain and heart disease medicine from one or several tanshinone IIA, medical salt, Notoginsen triterpenes, carthamus tinctorius yellow colour, haw leaf total flavone, puerarin and lamp-dish flower element; choosing tanshinone IIA or medical salt as tanshinone IIA mahogany. This invention can used to treat coronary disease, angina pectoris and so on.
Owner:JIANGYIN TIANJIANG PHARMA
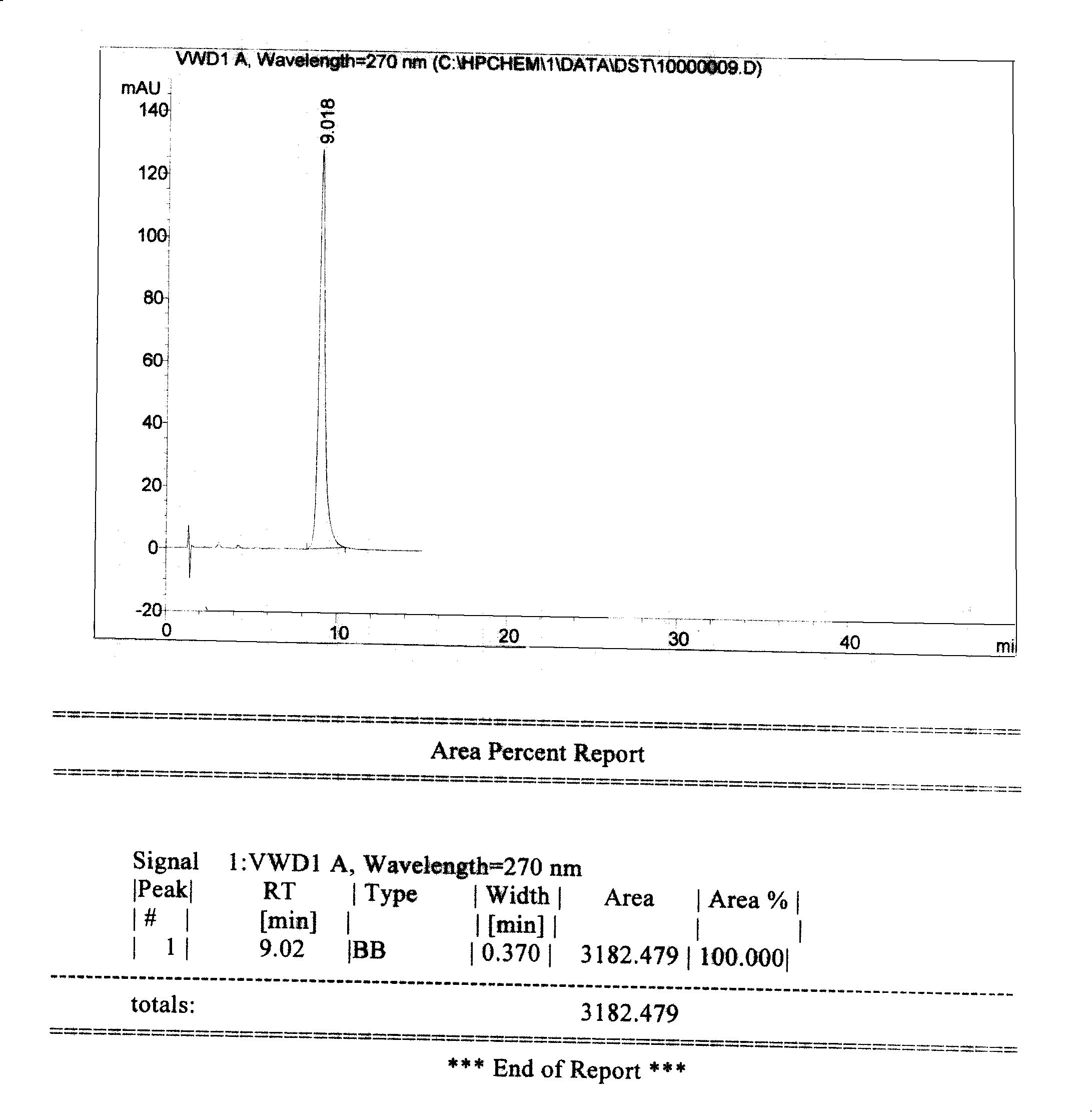
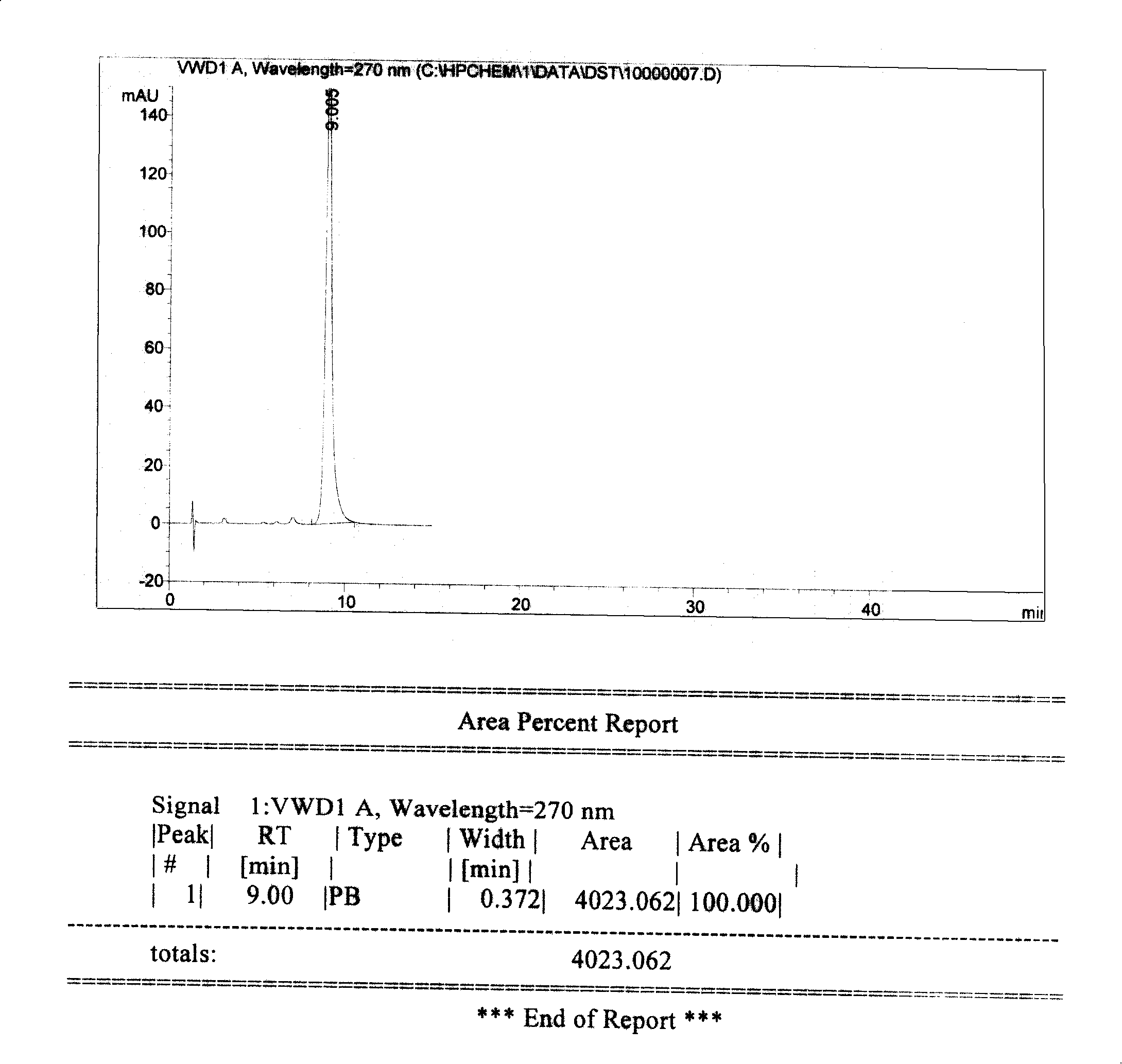
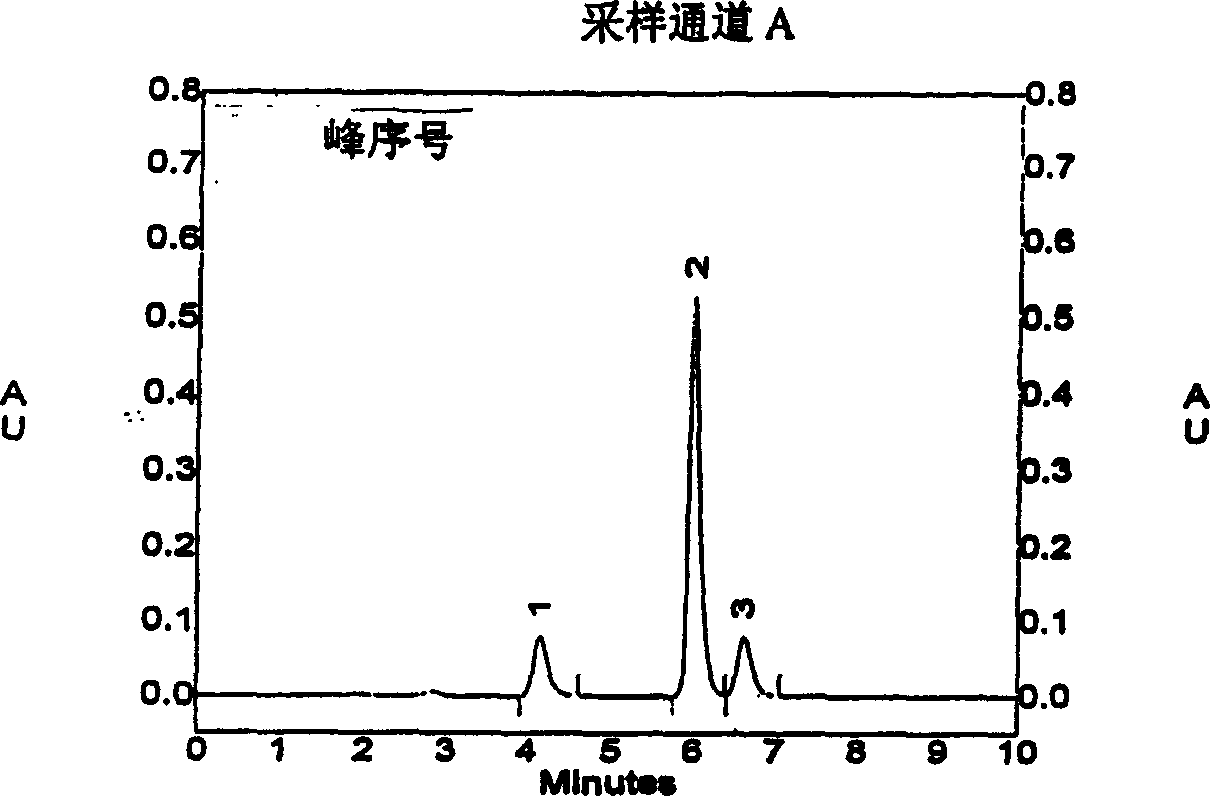
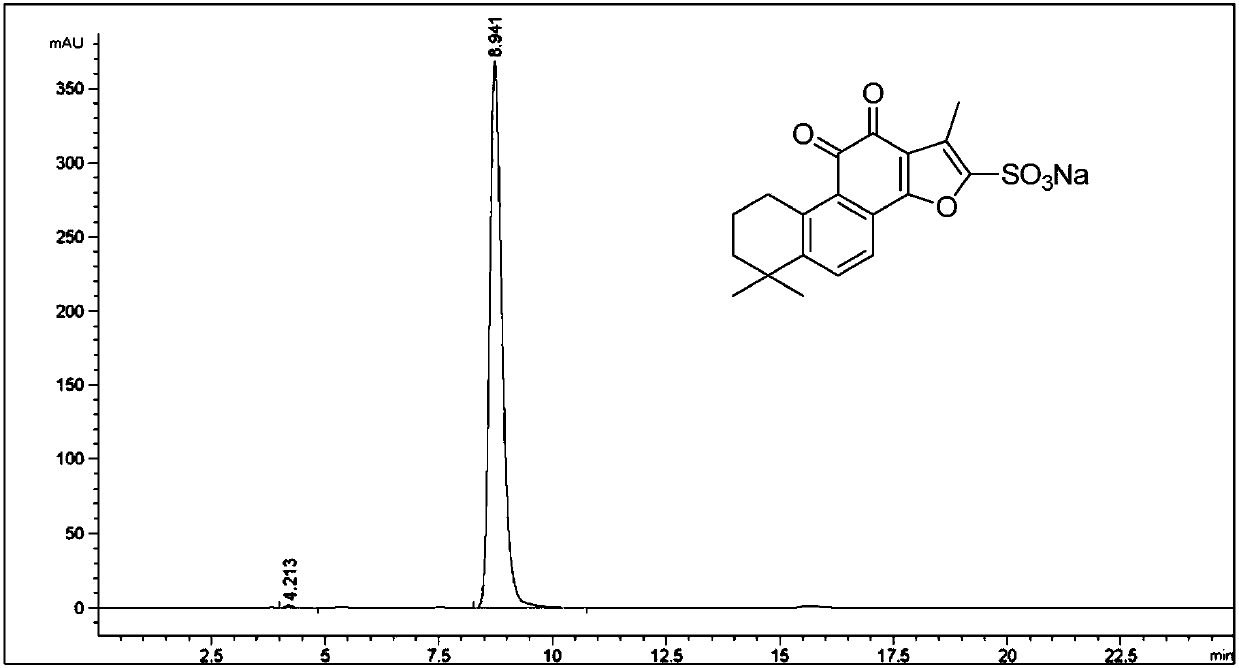
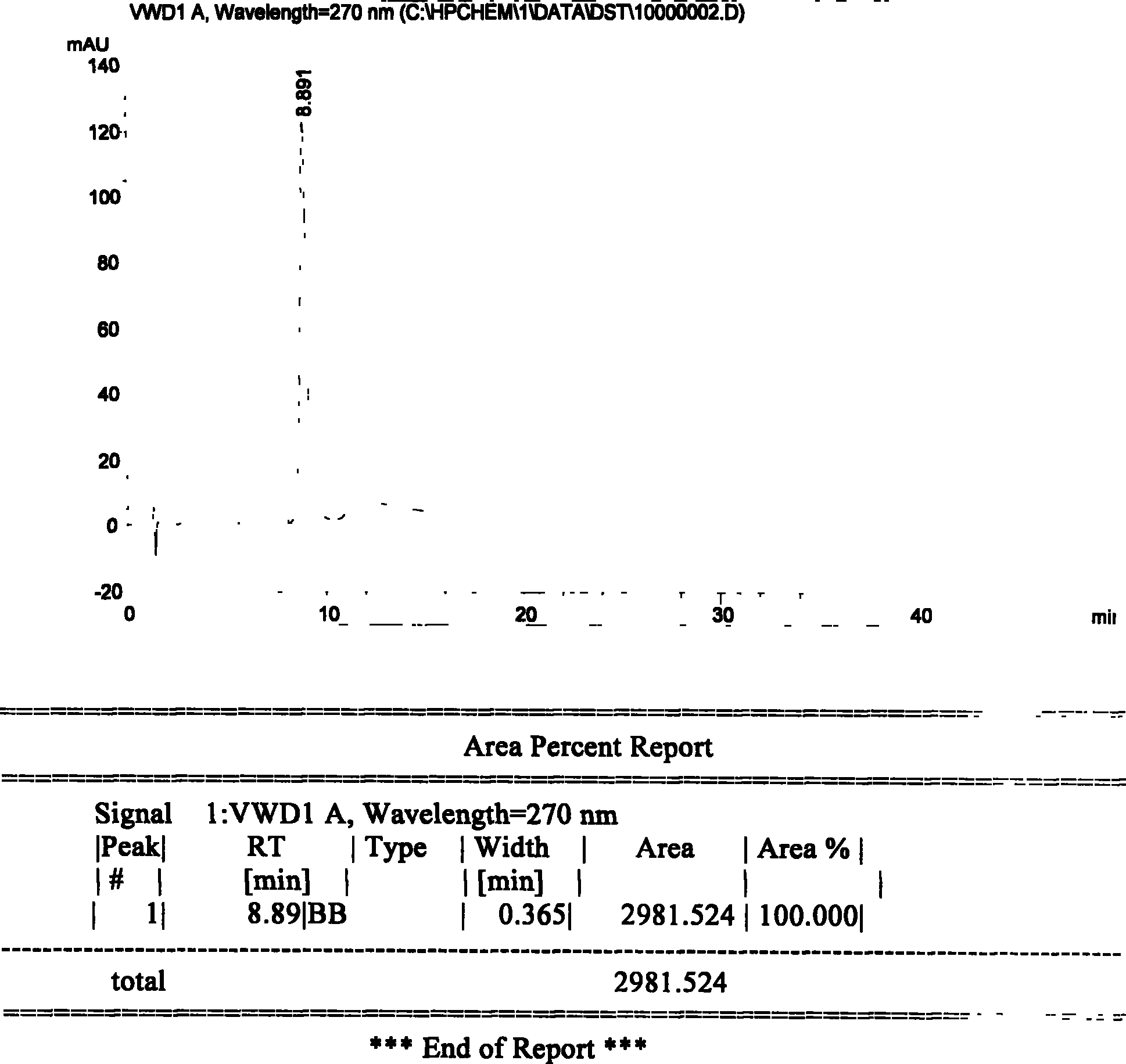
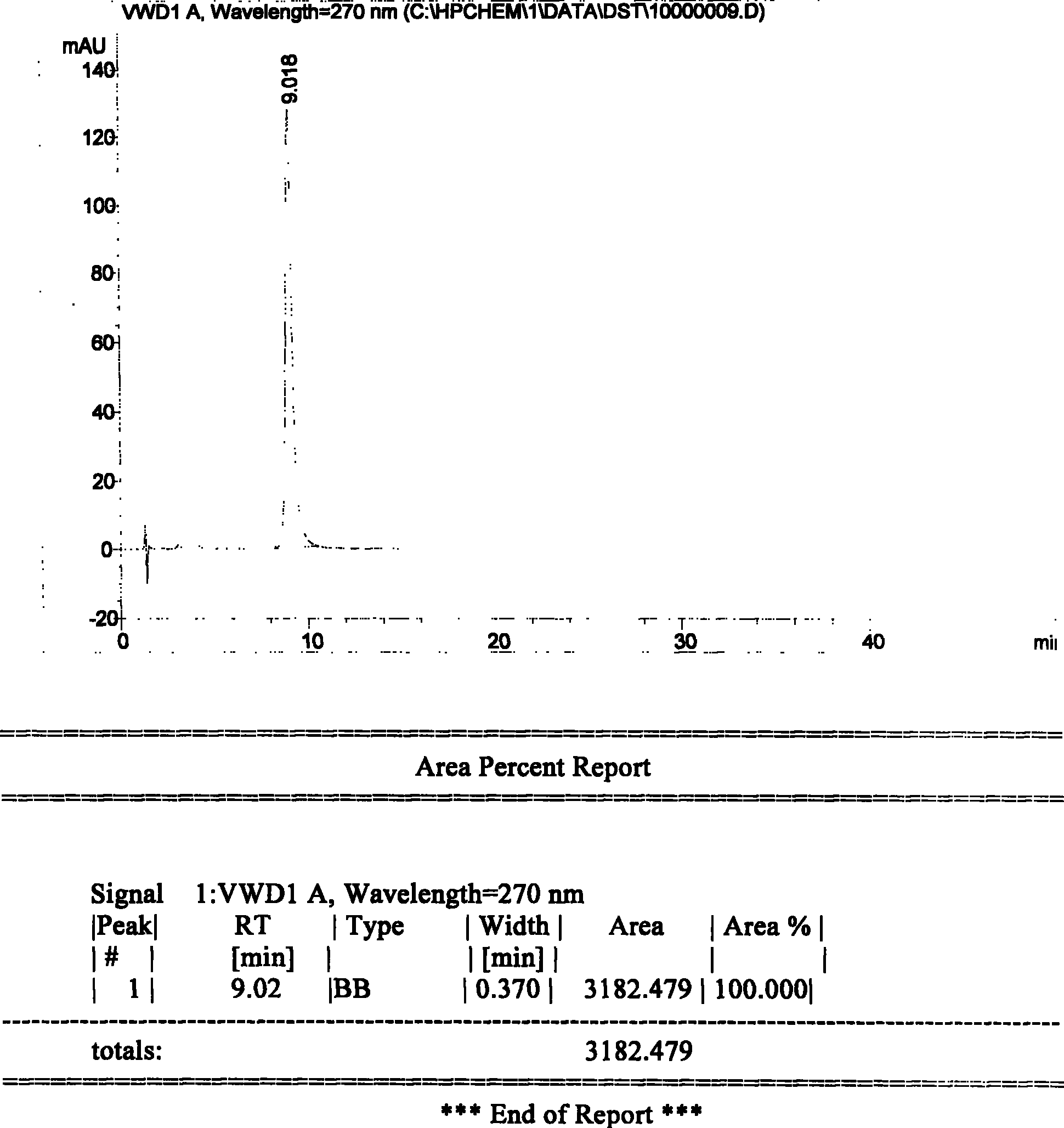
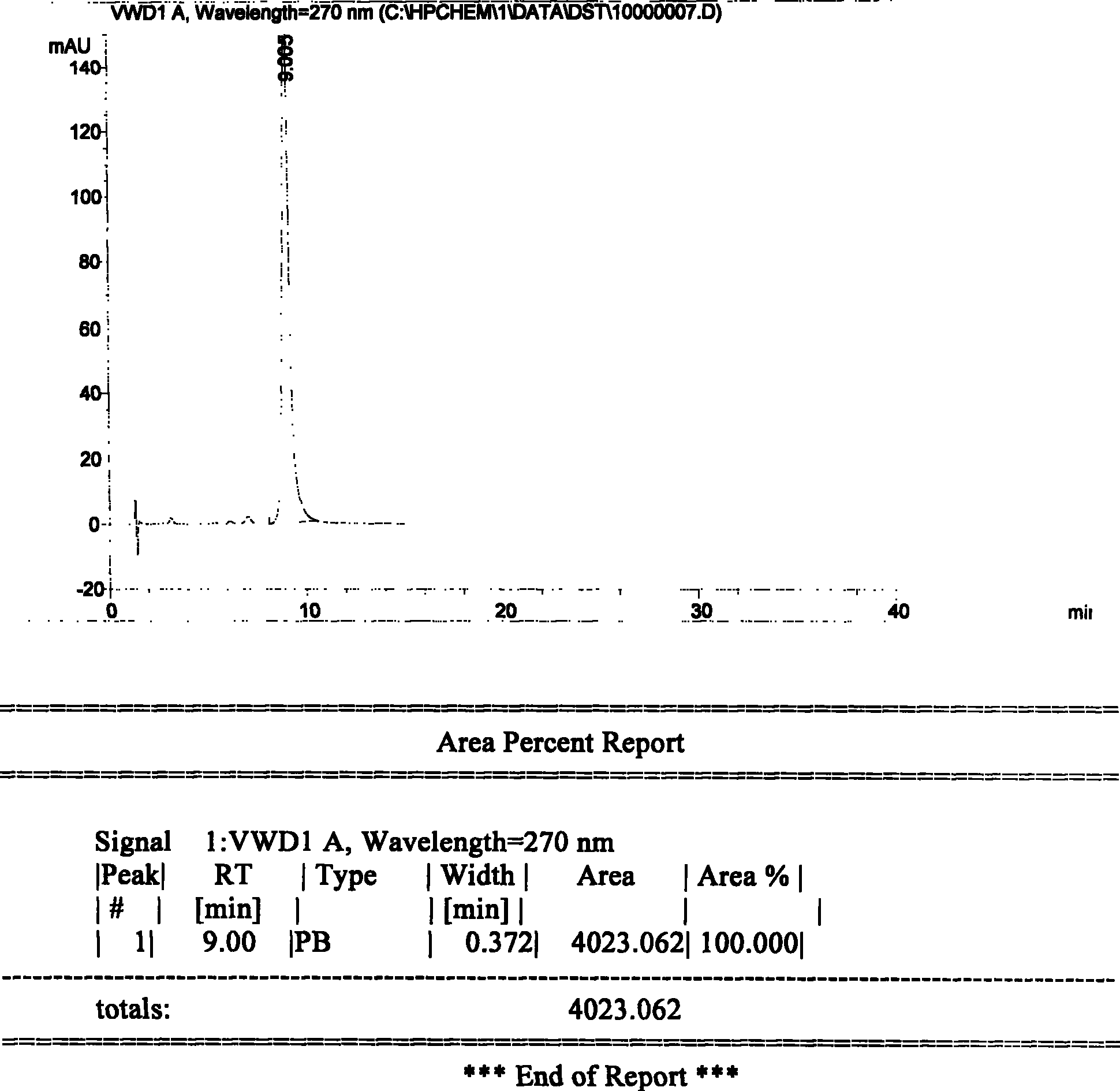
A high efficient liquid phase analysis method of sodium tanshinone IIA sulfonate
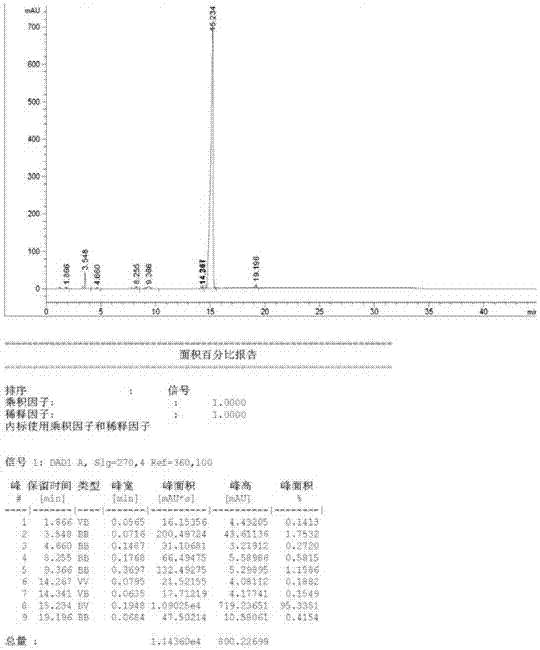
The invention is a high performance liquidoid analytical method of tanshinone IIA sodium sulfonate which employs the reverse-phase gradient elution method, The proportion of organic phase and aqueous phase is 0-80:20-40 at 0 minute -8 to 20 minutes,the proportion of organic phase and aqueous phase is 80-95:5-20 at 8 minutes to 20 minutes -20 to 40 minutes. The invention can detect the tanshinone IIA, tanshinone I sodium sulfonate and other relative substance in tanshinone IIA sodium sulfonate at the same time, resolve the shortage of general liquid phase analysis, effectively control the quality of raw material of tanshinone IIA sodium sulfonate and its preparation, quickly and conveniently detect the relative substance of raw material of tanshinone IIA sodium sulfonate and its preparation.
Owner:JIANGSU CAREFREE PHARM CO LTD
Injection liquid of sodium tanshinon IIA silite
InactiveCN1994292AInhibit oxidative decompositionSimple production processOrganic active ingredientsPharmaceutical delivery mechanismCoronary artery diseaseAngina
The invention relates to a sodium tanshinone IIA silate injection which comprises the constituents of (by weight percent) tanshinone II A sodium sulfonate 0.1-99. 9%, anti-oxidant 0.1-99. 9%. The medicament can be administered through intramuscular injection or venous instillation, and is mainly used for treating coronary disease, angina pectoris and pain of the chest.
Owner:广东宏远集团药业有限公司 +1
Tanshinone II A sulfonate, preparation method, and applications thereof
InactiveCN103450325ASimple and fast operationEase of industrial productionOrganic active ingredientsSteroidsSodium tanshinone IIA sulfonateAqueous solution
The invention discloses a tanshinone II A sulfonate, and a preparation method thereof. The preparation method comprises following steps: (1) dissolving crude tanshinone II A sulfonate in a methanol water solution with a concentration of 15% to 95% and a temperature of 50 DEG C to 80 DEG C, carrying out stirring absorption with an absorbing agent, controlling the solution temperature in the range of 50 DEG C to 80 DEG C during absorption and the stirring time in the range of 0.5 to 1.5 hours, and filtering when the solution is still hot; (2) subjecting the solution obtained in the step (1) to a reduced pressure condensation treatment until the volume of the condensed solution is 40% to 60% of the original volume; (3) subjecting the solution obtained in the step (2) to a cooling treatment to separate out crystals, and controlling the devitrification temperature in the range of minus 5 DEG C to 10 DEG C, and the devitrification time in the range of 5 to 20 hours; (4) drying the crystals obtained in the step (3). The invention also discloses applications of the tanshinone II A sulfonate. The preparation method can prominently improve the quality of tanshinone II A sulfonate, and prominently reduce clinical potential risk of injections made of tanshinone II A sulfonate.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
Sodium tashinone II A sulfonate injection and preparation method thereof
InactiveCN1875925AHigh yieldOrganic active ingredientsInorganic non-active ingredientsTanshinone IIASodium tanshinone IIA sulfonate
The invention relates to a sodium tanshinone IIA silate injection and process for preparation, wherein the raw material comprises superfine sodium tanshinone IIA silate and medicinal auxiliary materials for injections. The injection has the advantages of high yield and good stability.
Owner:JILIN SIHUAN PHARM CO LTD
Pharmaceutical composition of salvia miltiorrhiza extractive, and use thereof
The invention relates to a pharmaceutical composition of salvia miltiorrhiza bge extractive, and the use of the pharmaceutical composition. The pharmaceutical composition is formed by combining salvianolic acid A or salvianolic acid B with tanshinone IIA sodium sulfonate or combining salvianolic acid A and salvianolic acid B with tanshinone IIA or sodium tanshinone IIA sulfonate. The therapeutical effect on myocardial infarction of the pharmaceutical composition is better than that of each constituent used singly, and the pharmaceutical composition can better achieve the therapeutical effects of multiple ingredients and multiple target points of salvia multiorrhiza bge and is used for preparing medicine for treating cardio-cerebrovascular diseases. According to the conventional manufacturing technology of pharmaceutics, the pharmaceutical composition can be processed into tablets, capsules, granules, freeze-drying preparation for injection, or freeze-drying preparation or powder for injection of carboxylate of the pharmaceutical composition.
Owner:SHANDONG UNIV
Sodium sulfonate injection of tanshinone óÄA, and preparation method
An injection of tanshinone IIA-sodium sulfonate with high effect and stability contains tanshinone IIA-sodium sulfonate and hydroxypropyl-beta-dextrin in weight ratio of 1: (1-150). its preparing process is also disclosed.
Owner:JIANGSU CAREFREE PHARM CO LTD
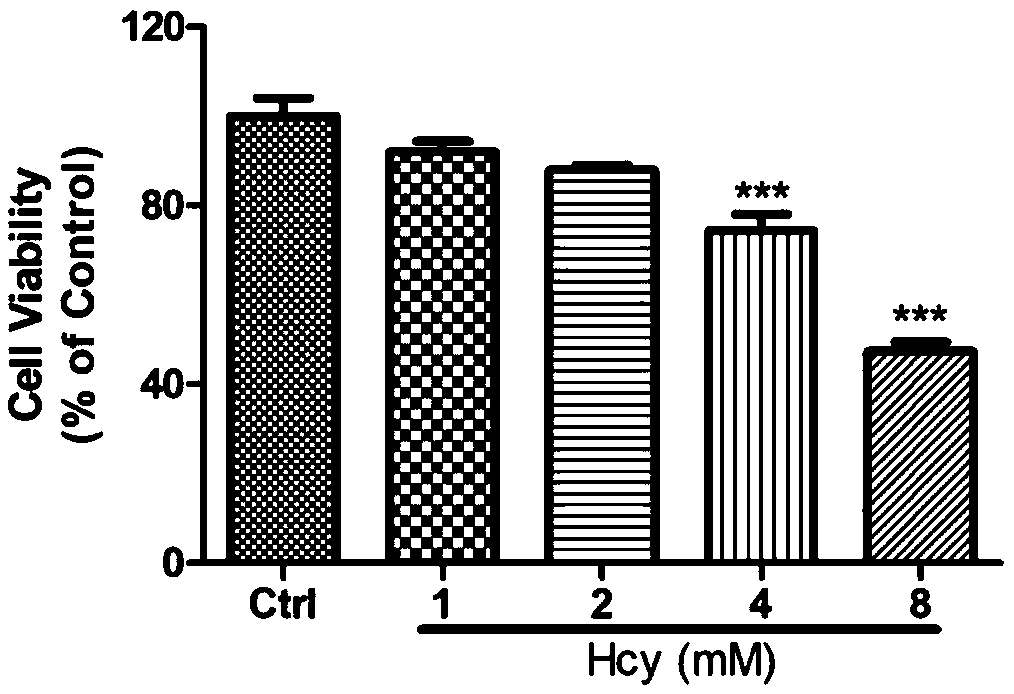
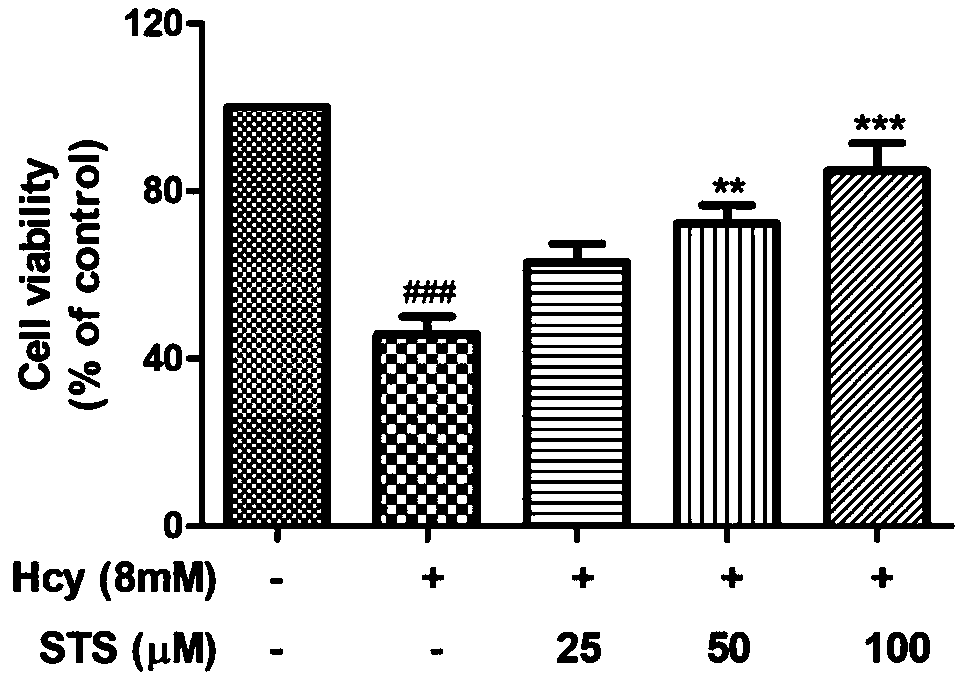
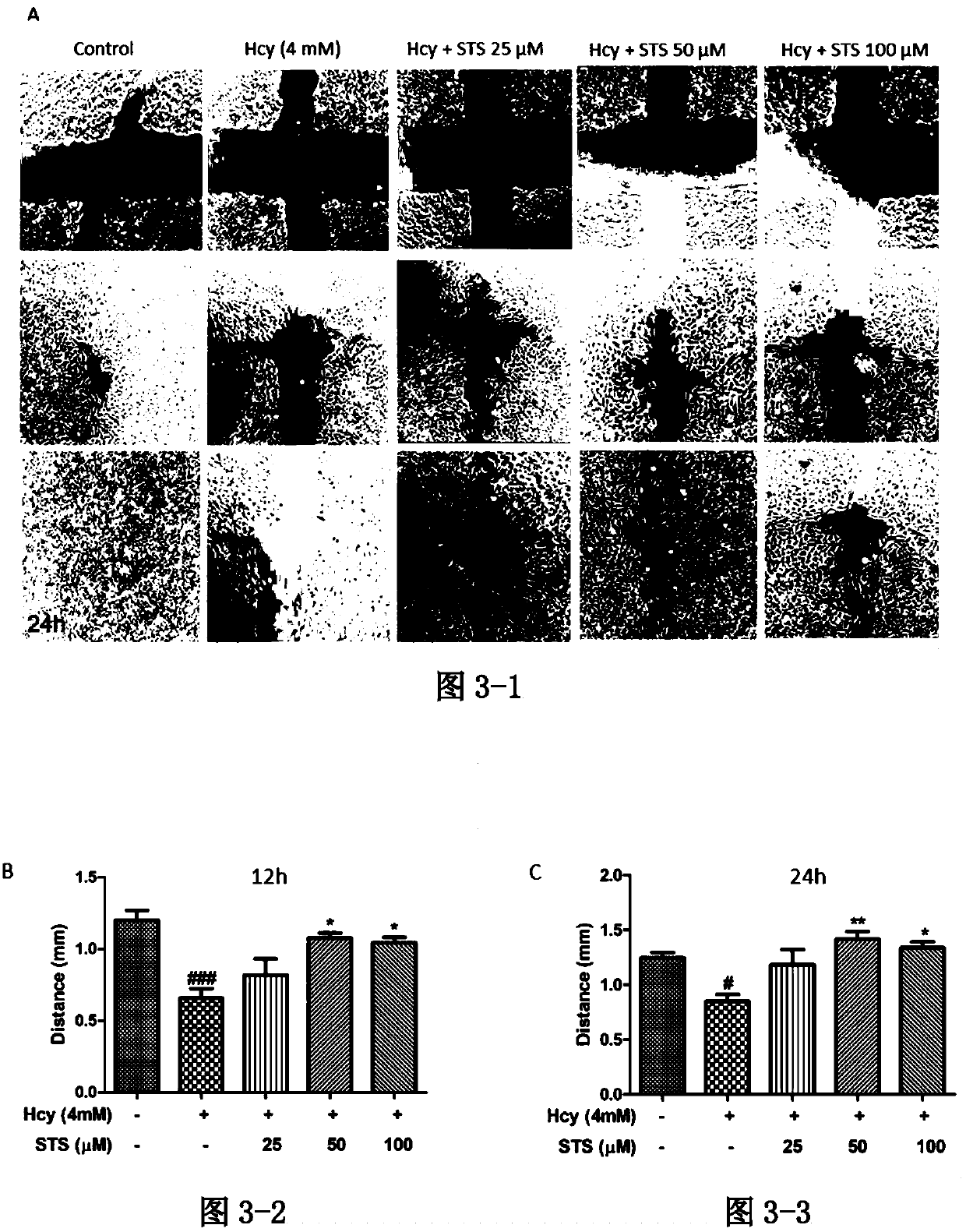
Application of sodium tanshinone IIA sulfonate in preparation of medicines for treating acute or chronic diseases accompanied with increase of homocysteine in blood
PendingCN110893187AHighly toxicAvoid damageOrganic active ingredientsSenses disorderVascular endotheliumBlood vessel injury
The invention relates to application of sodium tanshinone IIA sulfonate in preparation of medicines for treating acute or chronic diseases accompanied with increase of homocysteine in blood, alone oras an effective component. Vascular endothelial cells, myocardial cells, nerve cells and isolated blood vessel models are used to simulate the damage effect of the homocysteine on cardiovascular and cerebrovascular systems, it discovers that sodium tanshinone IIA sulfonate has a protection and / or treatment effect on the cardiovascular and cerebrovascular damages caused by the homocysteine, and theblank of protection and / or treatment medicines for resisting the cardiovascular and cerebrovascular damages caused by the homocysteine is filled up. The medicines provided by the invention are suitable for diseases causing abnormal increase of the homocysteine in the blood, can play a role in protecting and / or treating cardiovascular and cerebrovascular injuries caused by resisting the homocysteine only by reaching effective concentration in the blood, and provide a better choice for treating the cardiovascular and cerebrovascular injuries of diseases causing the increase of the homocysteinein the blood.
Owner:LONGHUA HOSPITAL SHANGHAI UNIV OF TRADITIONAL CHINESE MEDICINE
Injecting tanshinone II and its derivative concentrated solution and its production
InactiveCN101073555ASolve instabilitySolve instability bugsAntibacterial agentsOrganic active ingredientsTanshinone IIATashinone IIA
The invention is concerned with the injecting thick solution and the preparation method that prepares by Tanshinone IIA or Sodium Tanshinone IIA.
Owner:秦长青
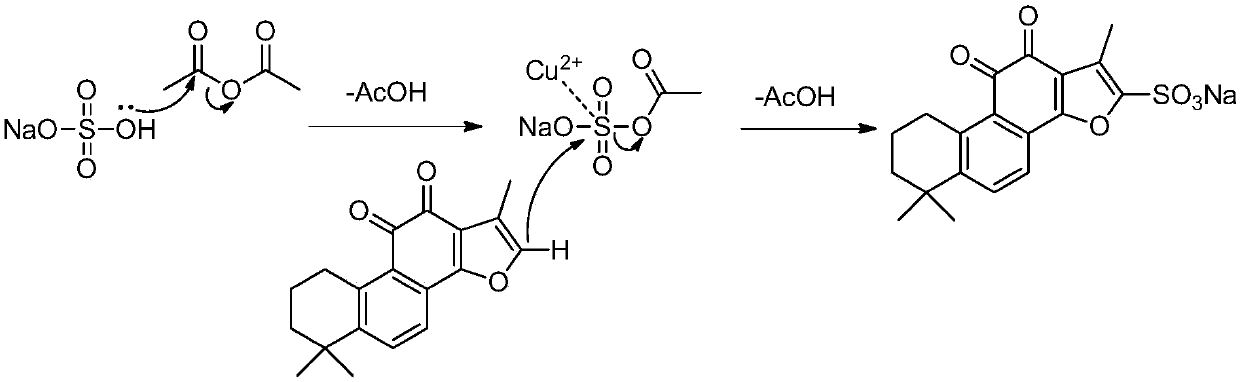
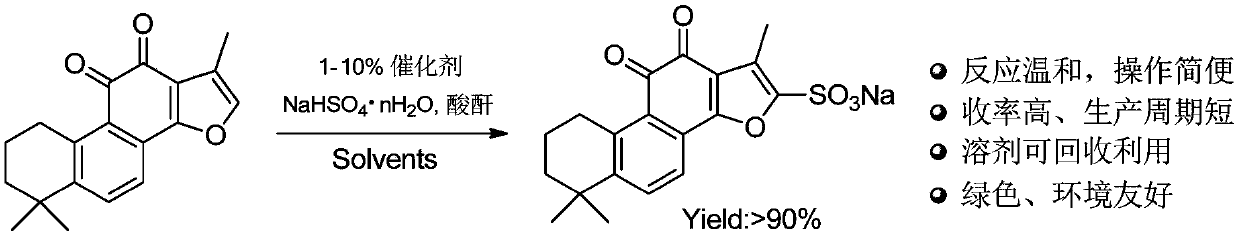
Preparation method for green synthesis of tanshinone IIA sodium sulfonate
The invention discloses a novel green synthesis method of tanshinone IIA sodium sulfonate, which comprises the following step: directly carrying out a sulfonation reaction on tanshinone IIA by adopting safe, stable and cheap sodium hydrogen sulfate in the presence of a catalyst to obtain tanshinone IIA sodium sulfonate. Compared with the existing method, the method has the advantages that a targetproduct can be obtained through a one-step reaction; strong corrosive sulfonation reagents such as concentrated sulfuric acid and pyridine sulfur trioxide and a large amount of saturated salt water and sodium carbonate needed by subsequent salt forming are avoided. The method has the advantages that the production process is simple and convenient, the efficiency is high, and reagents can be recycled, so that the production cost of the tanshinone IIA sodium sulfonate is obviously reduced, the environmental pollution pressure is greatly relieved, and the method has a great industrial productionvalue.
Owner:SHANGHAI XINGYE PHARM TECH CO LTD
Injection in large capacity of sodium sulfonate containing tanshinone óÄA and preparation method
InactiveCN1915230AImprove pharmaceutical performanceMeet the requirements for intravenous drugsOrganic active ingredientsPharmaceutical delivery mechanismSulfonateTanshinone IIA
A high-capacity injection contains tanshinone IIA-sodium sulfonate and pharmacologically acceptable additive. Its preparing process is also disclosed.
Owner:JILIN SIHUAN PHARM CO LTD
Sulfotanshinone IIA derivatives, and synthesis and applications thereof as drug
ActiveCN106478765AKeep aliveGood water solubilityOrganic active ingredientsSenses disorderDiseaseSolubility
The invention discloses sulfotanshinone IIA derivatives, and synthesis and applications thereof as a drug. Specifically, the invention relates to novel derivatives represented by the formula (I) and pharmaceutically acceptable salts of the derivatives, a pharmaceutical composition comprising the same, and a preparation method thereof. The invention also discloses applications of the derivatives in the preparation of drugs for treating coronary heart disease, angina pectoris, myocardial infarction, viral myocarditis, arrhythmia, cerebrovascular disease, hepatitis, pulmonary heart disease, bronchial asthma, tumors, kidney diseases, eye diseases, vasculitis with artery occlusion, hypertension, bone fracture, burns, surgical operation, Bechcets syndrome, and the like. The derivatives have the advantages that the activity of tanshinone IIA is maintained, and the water solubility of tanshinone IIA is improved. Compared with sodium sulfotanshinone IIA, the stability is improved, the acidity of the derivatives is prominently reduced, and the irritancy in injection is avoided. The definitions of substituents of the formula (I) are the same as the definitions in the description.
Owner:北京桦冠医药科技有限公司
Pharmaceutical composition of salviae miltiorrhizae extract for treating cardiovascular and cerebrovascular diseases and use thereof
The invention relates to a pharmaceutical composition of salviae miltiorrhizae extract for treating cardiovascular and cerebrovascular diseases and use thereof. The pharmaceutical composition is a combination of danshinolic acid A or danshinolic acid B and tanshinone IIA sodium sulfonate, or a combination of danshinolic acid A, danshinolic acid B and tanshinone IIA or tanshinone IIA sodium sulfonate. The treatment effect of the pharmaceutical composition is better than that of single application of the ingredients on myocardial infarction, the treatment effect of multi-component and multi-target of the traditional Chinese medicine salviae miltiorrhizae is better expressed, and the pharmaceutical composition is used for preparing drugs for treating cardiovascular and cerebrovascular diseases. The pharmaceutical composition can be processed to tablets, capsules, granules, freeze-dried preparations for injection or freeze-dried preparations for injection or powder-injection of carboxylate according to routine production process of pharmaceutics.
Owner:SHANDONG UNIV
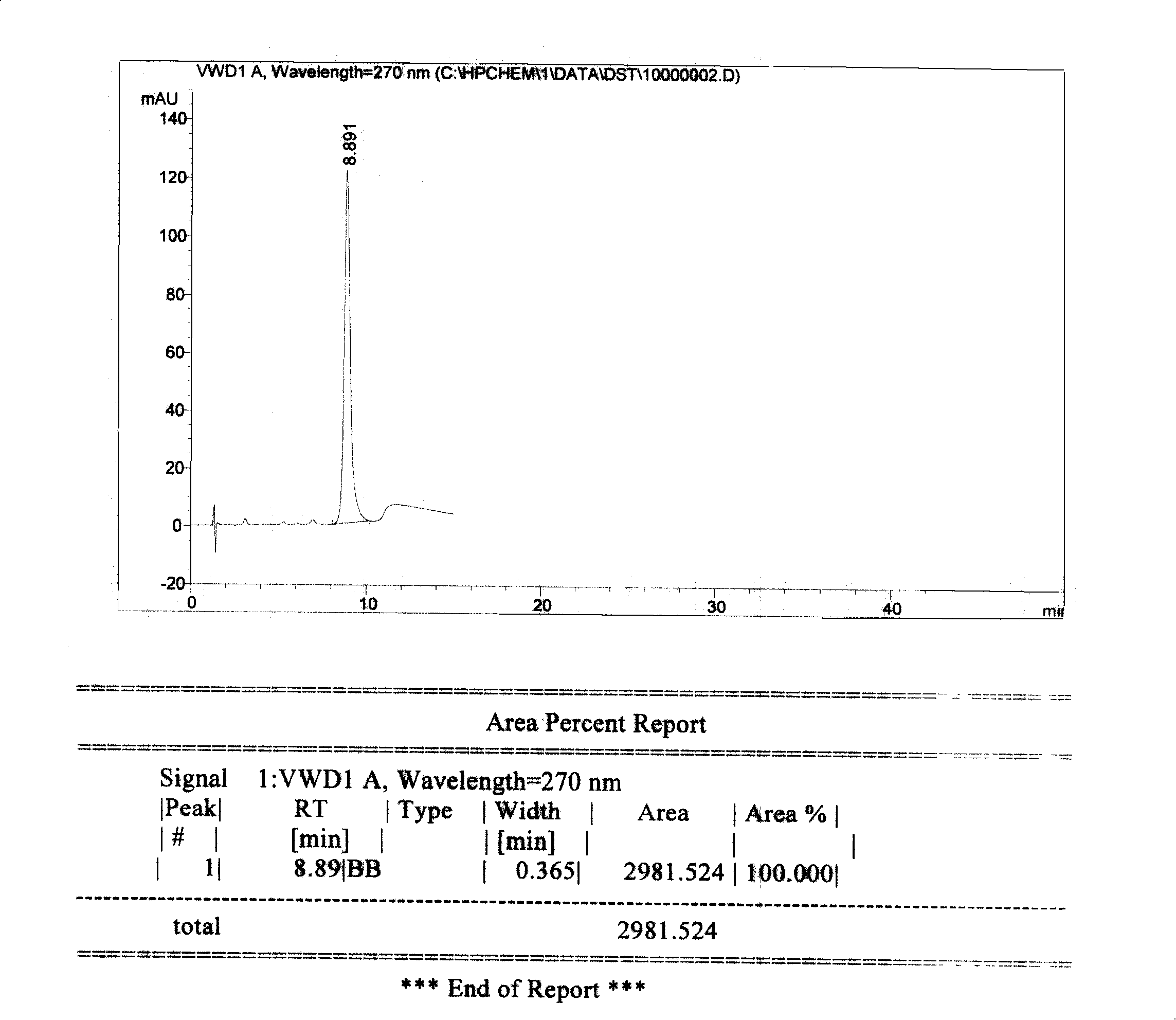
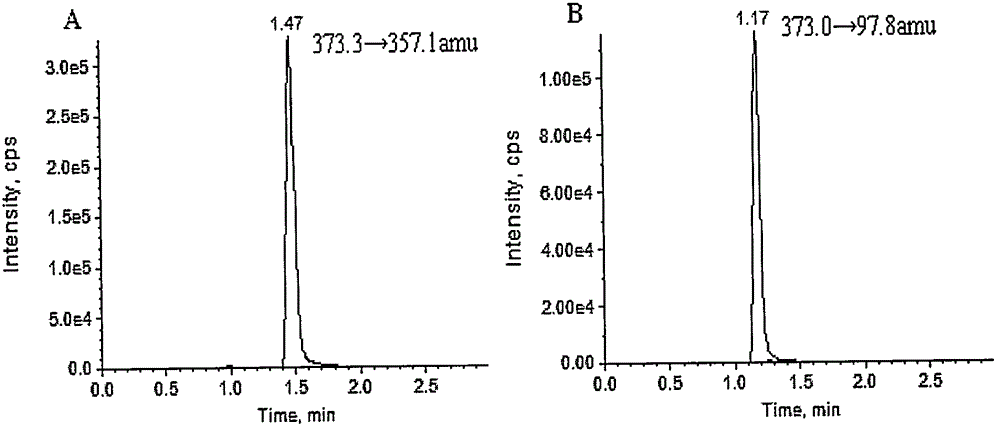
Method for measuring concentration of Sodium Tanshinone IIA Sulfonate (STS) in human plasma
InactiveCN105301155AReduce dosageOptimize mobile phase conditionsComponent separationUltravioletBlood plasma
The invention belongs to the field of medical examination, and relates to the analysis and measurement method of in vivo drugs, particularly to a method capable of measuring the concentration of Sodium Tanshinone IIA Sulfonate (STS) in human plasma. The method uses deuterium 5-dehydroepiandrosteronesulfate (DHEAS-D5) as an interior label, and the condition of a yellow light safety lamp without UV (light at the wavelength of 420 nm or below is removed) is adopted to control STS degradation during the blood sample treatment process, so that the accuracy of the method is ensured; after the blood sample is acidized using formic acid, a certain amount of organic solvent methyl alcohol and acetonitrile mixed liquor is added to enable protein to precipitate; a tandem mass spectrometry is used to measure the concentration of STS and the interior label; the quantitative linear range is 2-1000 ng / mL, the requirements of human pharmacokinetic studies are met. The method has the advantages that the less sample is required, the pretreatment is simple, quick, and sensitive, only general-type equipment and reagents are required, the analysis period is short, and the cost is low; the method is applicable to the detection of clinical blood routine STS concentration.
Owner:AFFILIATED HUSN HOSPITAL OF FUDAN UNIV
Method for refining tanshinoneIIA sodium sulfonate
InactiveCN101200489BHigh purityComply with the requirements of raw materials for injectionSteroids preparationSulfonateOrganic solvent
The invention relates to a refining method of sodium tanshinone IIA sulfonate. The method comprises the following procedures, a) adding a crude sodium tanshinone IIA sulfonate to be purified into a mixture solution of an organic solvent and water, heating to 40 to 60 DEG C, agitating to dissolve and filtering to remove insoluble particles; b) slowly cooling the filtrate to the temperature of 0 to 30 DEG C and crystallizing and filtering; c) obtaining the sodium tanshinone IIA sulfonate with high purity after the decompression drying. The contents of the obtained sodium tanshinone IIA sulfonate can reach 99.5 percent and the quality can meet the purity requirement of a raw medicine for injection. The method of the invention is simple and is easy to operate with low refining cost, and is applicable for the industrialized production.
Owner:YAOPHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com