Patents
Literature
209 results about "Blood vessel injury" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
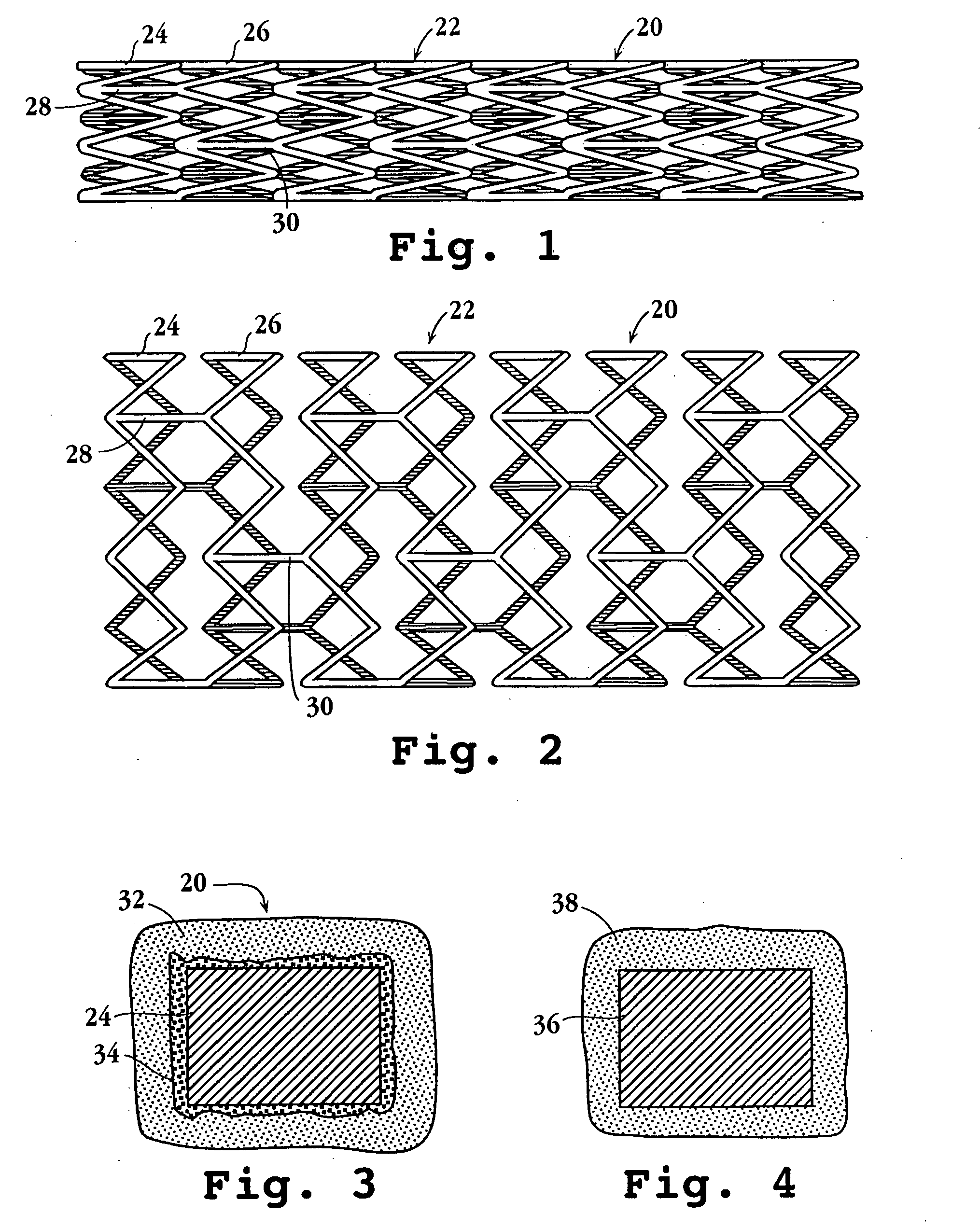
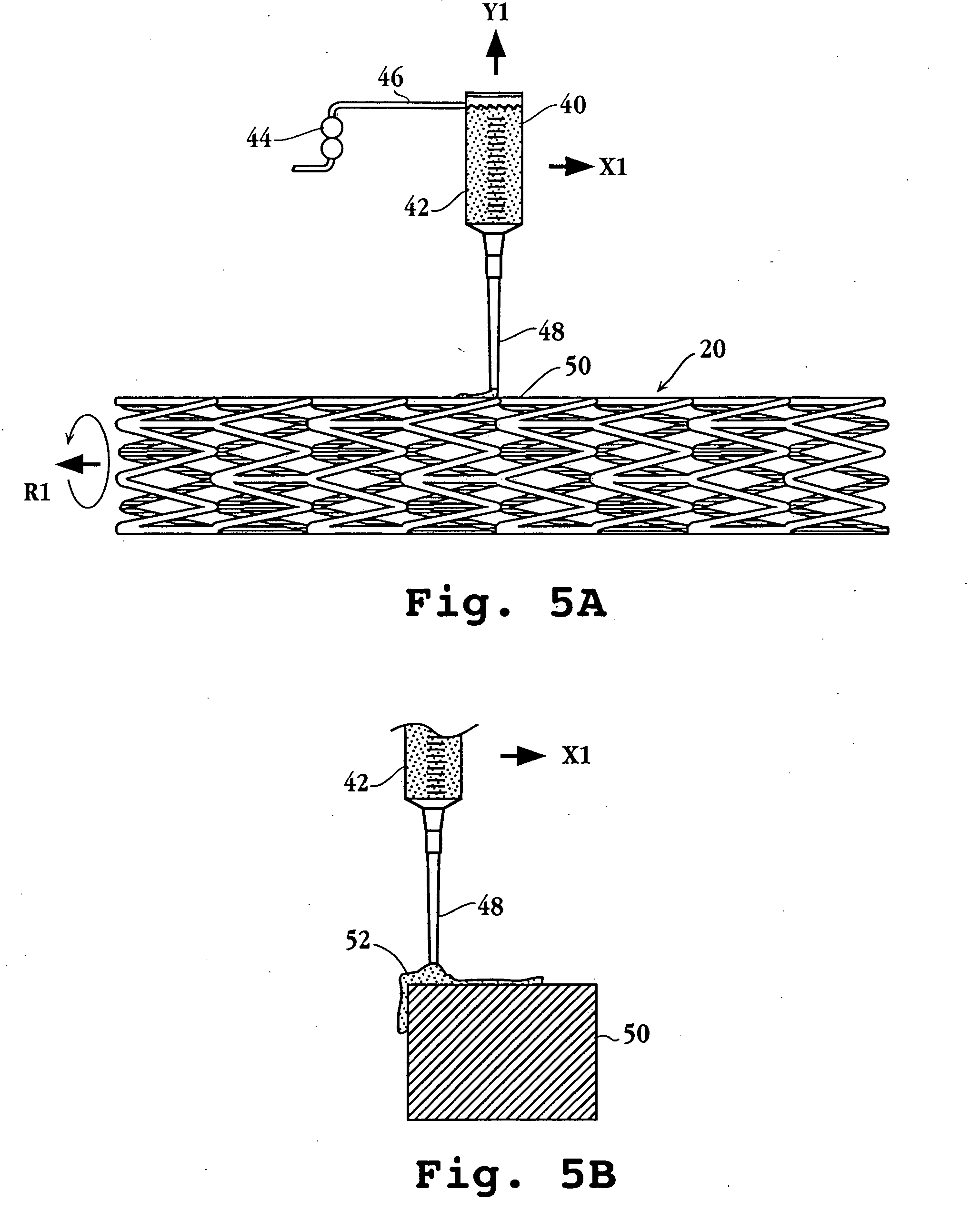
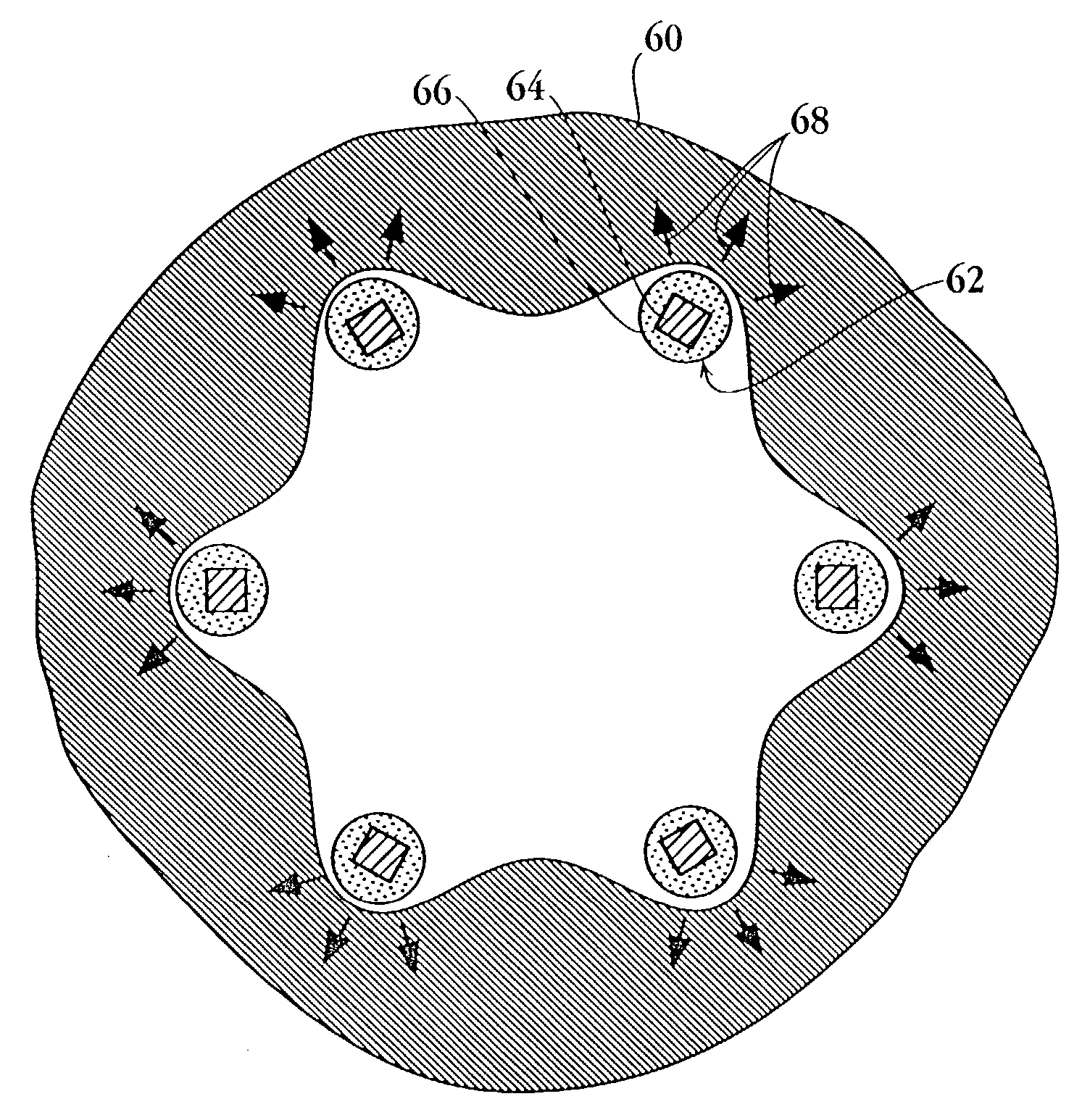
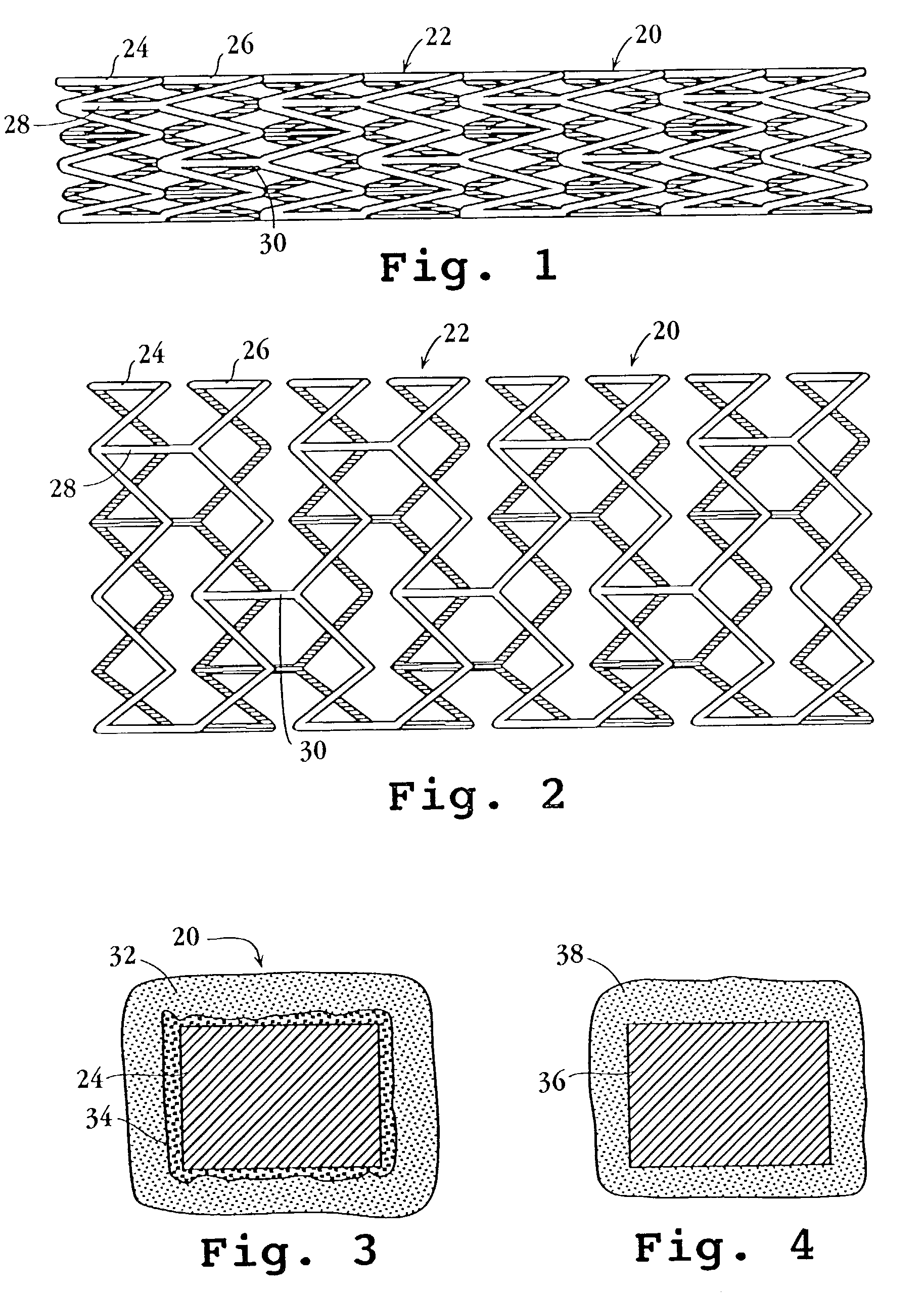
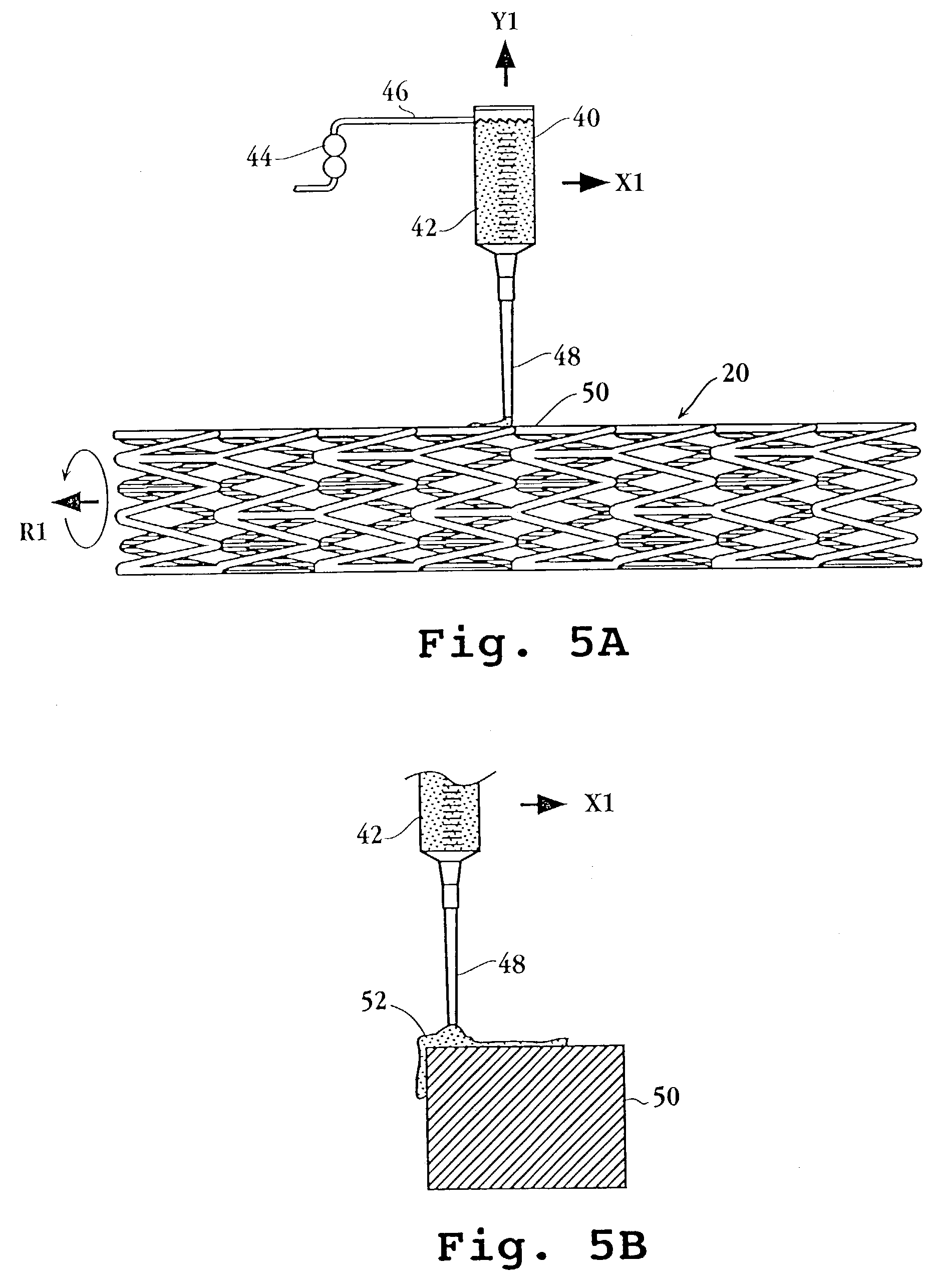
Drug-delivery endovascular stent and method for treating restenosis
InactiveUS6939376B2Efficient releaseOrganic active ingredientsOrganic chemistryRestenosisPoly dl lactide
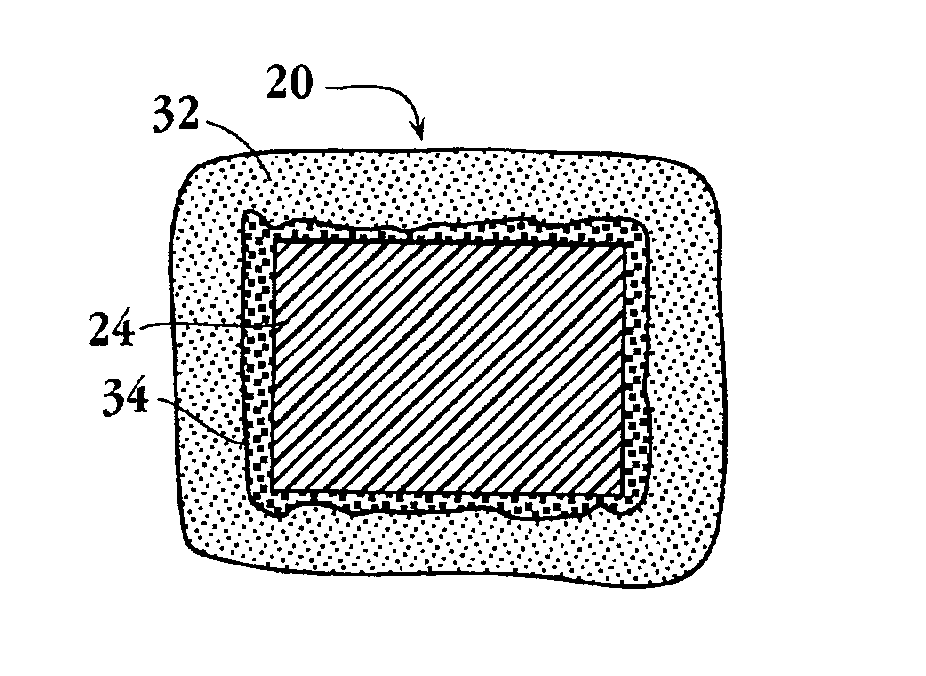
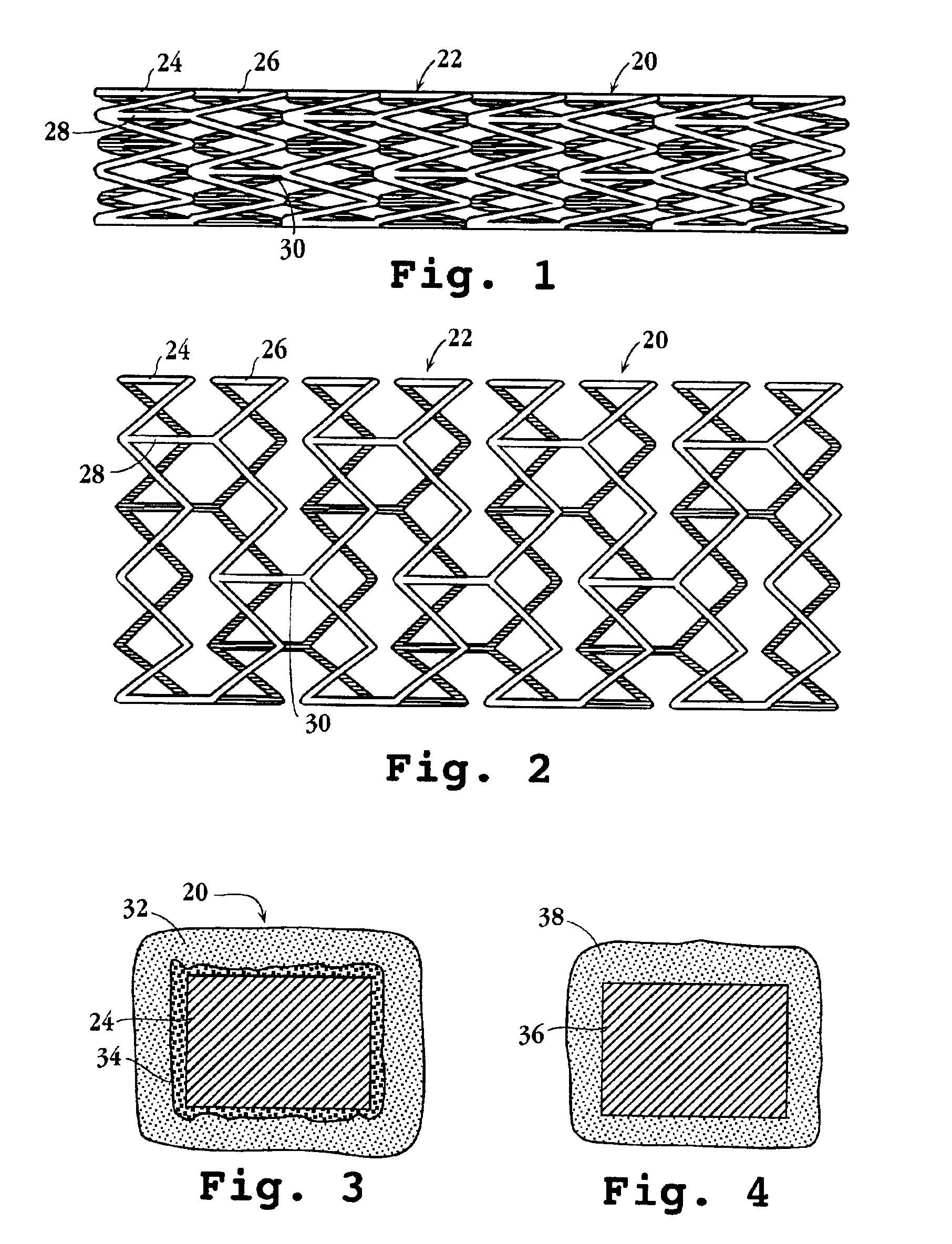
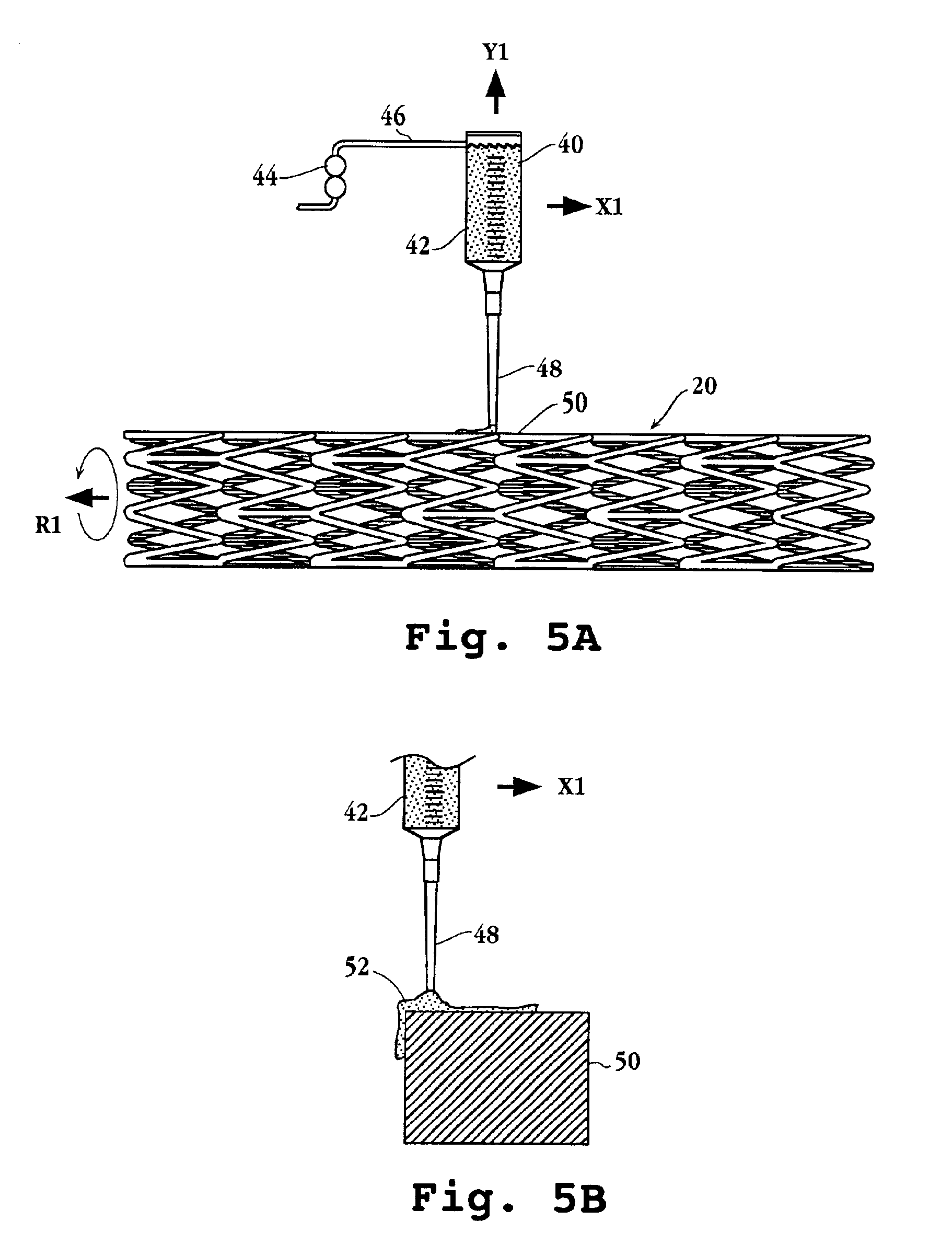
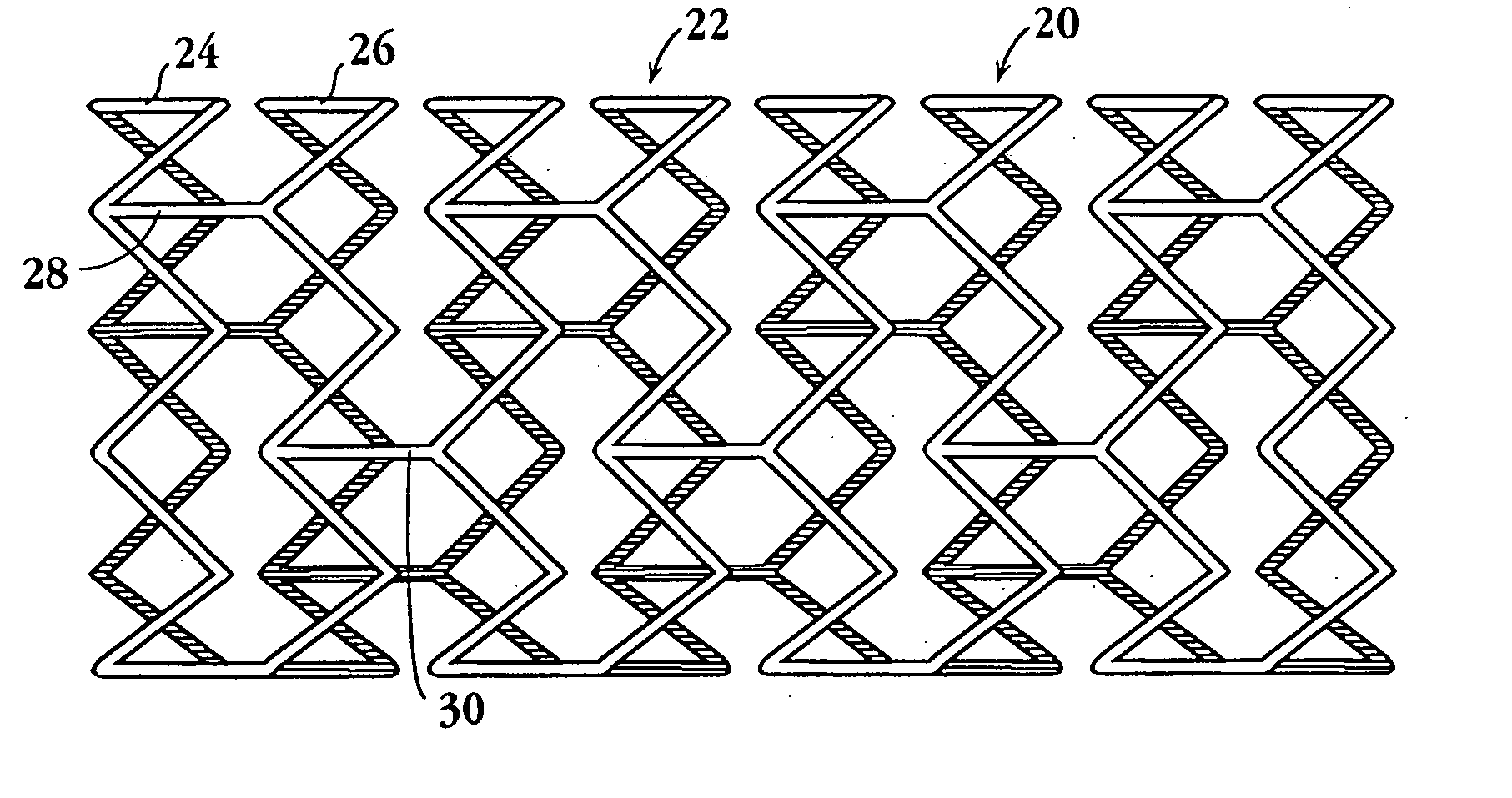
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
Drug-delivery endovascular stent and method of forming the same
ActiveUS20050038505A1Prevent restenosisEfficient releaseOrganic active ingredientsOrganic chemistryPolymer substrateInsertion stent
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of a monocyclic triene immunosuppressive compound having an alkyl group substituent at carbon position 40 in the compound. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-d / -lactide polymer substrate and (ii) 40-90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1-5 microns.
Owner:BIOSENSORS INT GROUP
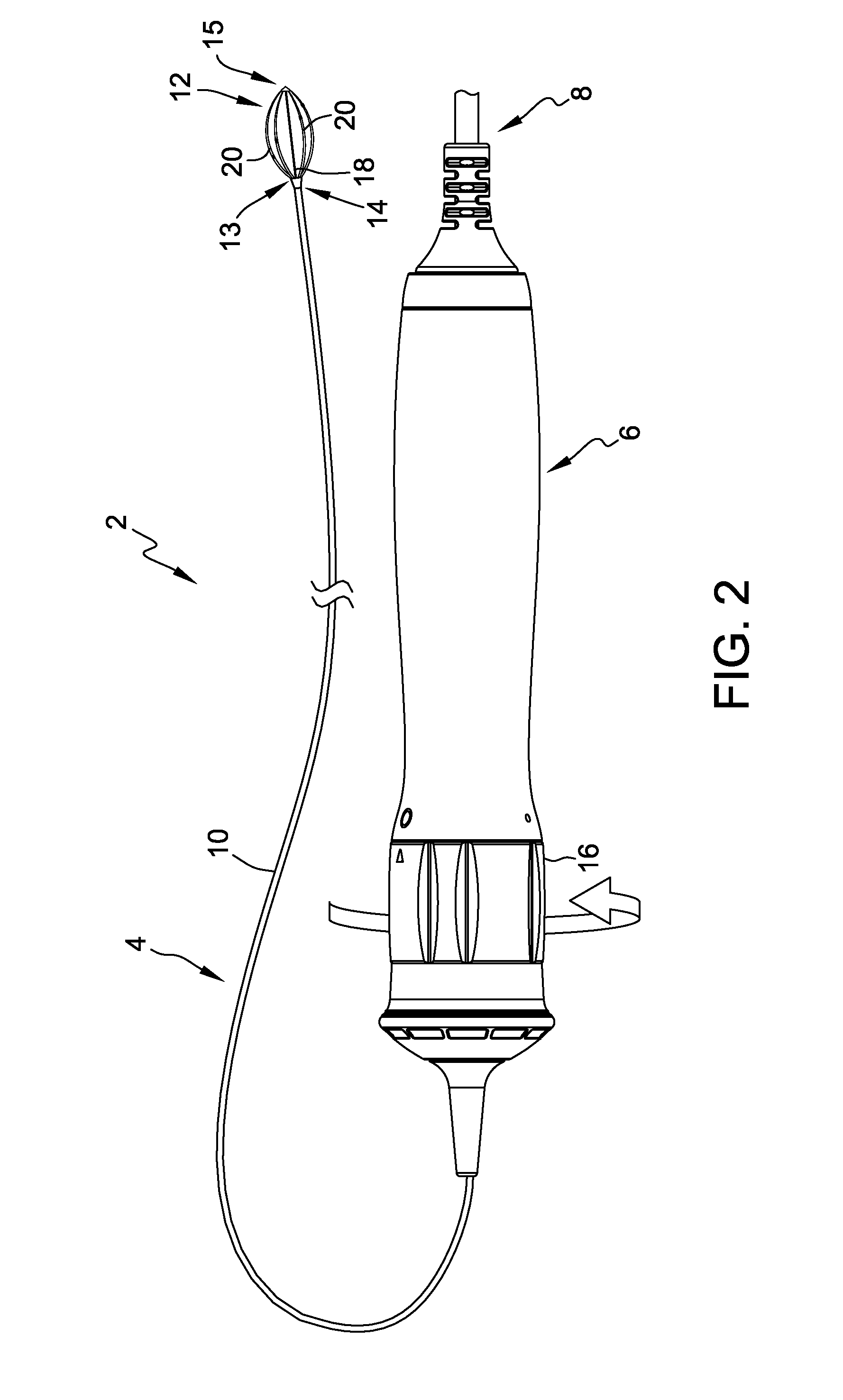
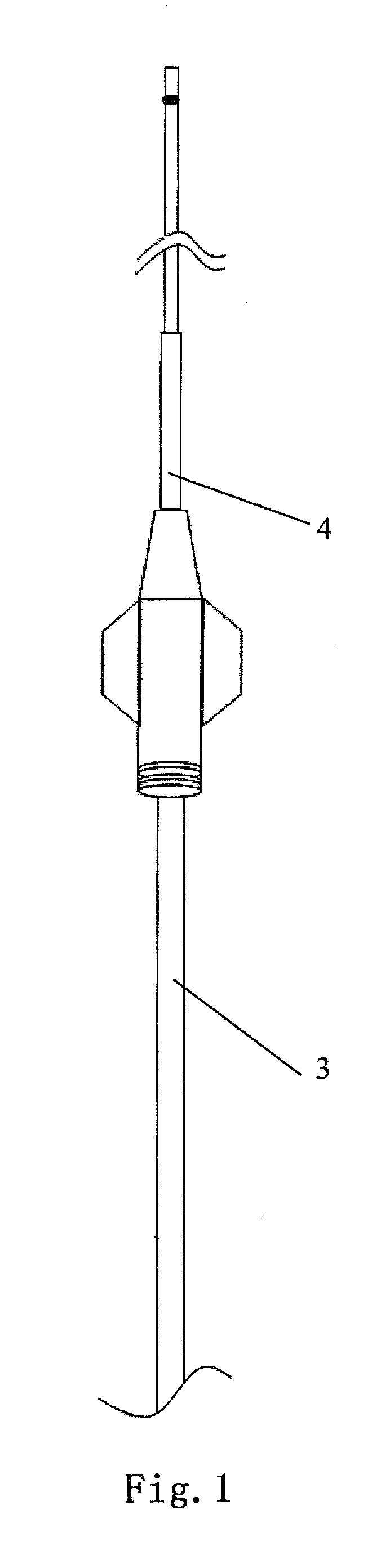
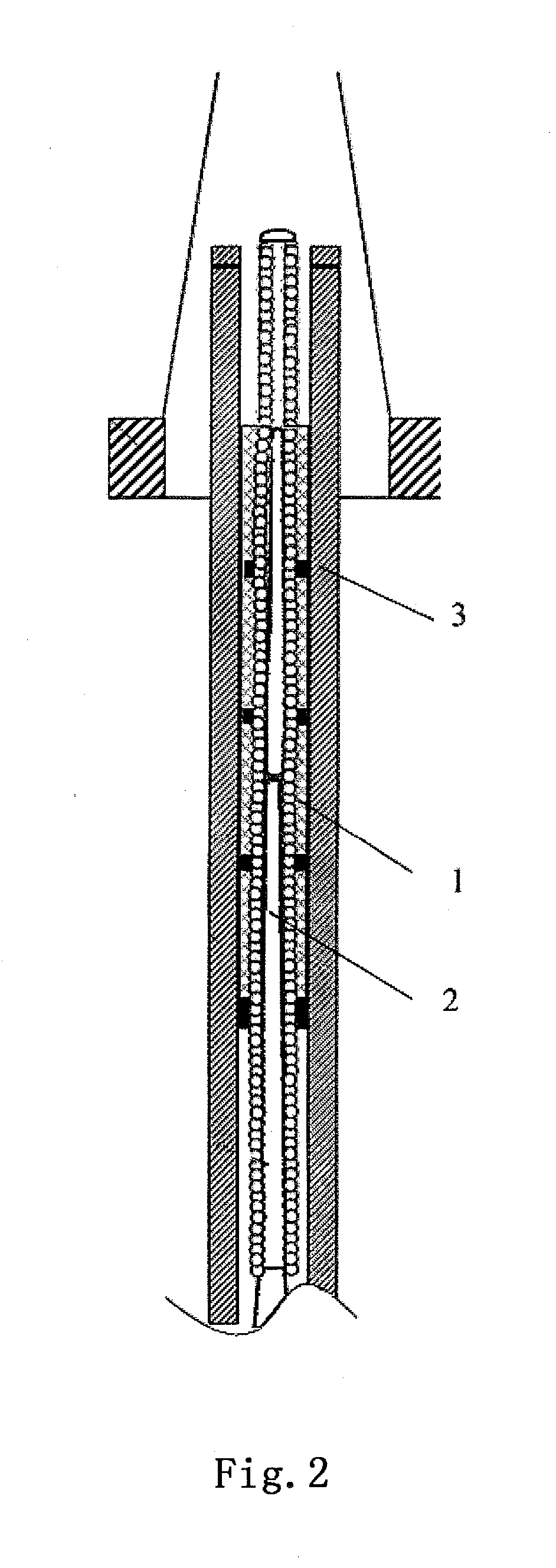
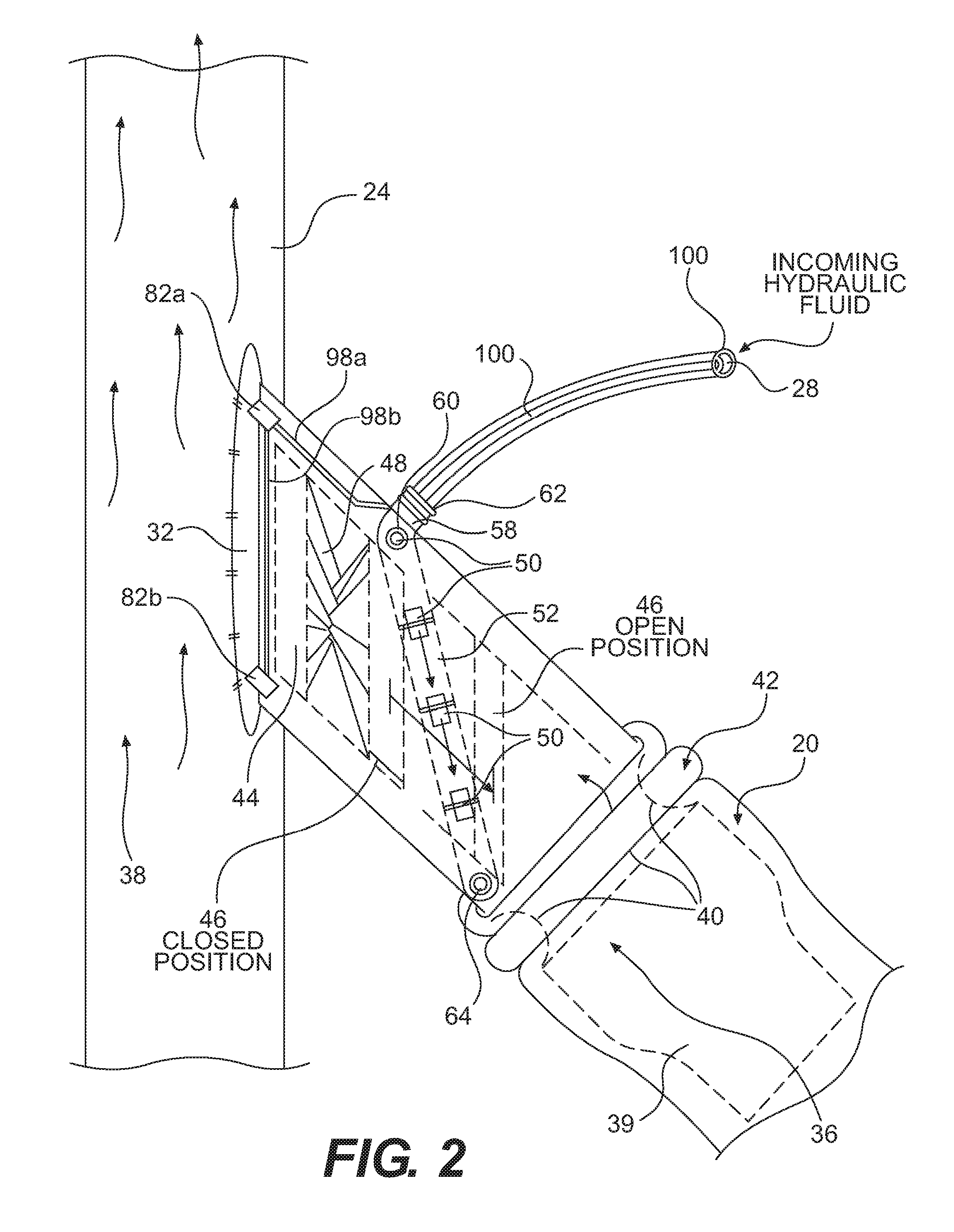
Flexible catheter shaft and method of manufacture
The present disclosure provides a strong, flexible catheter shaft for use in a catheter system. The flexible catheter shaft includes a braided polyimide tube including one or more outer layers of material of varying flexibility. The flexible catheter shaft provides a shaft having sufficient stiffness and kink resistance to allow an operator to advance an electrode basket connected to the flexible catheter shaft through a guide catheter to a target ablation site without causing vessel trauma. The distal tip of the flexible catheter shaft is designed to have sufficient flexibility to reduce any risk of kicking out a guide catheter when tracking the electrode basket around turns into side branches or bifurcations in the vasculature of a patient and includes a distal spring coil.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
System and method for in vitro blood vessel modeling
ActiveUS20110053207A1Reduce non-physiological shear stressMicrobiological testing/measurementLaboratory glasswaresCell layerVascular pathology
The present invention provides an in vitro blood vessel model for investigation of drug induced vascular injury and other vascular pathologies. The in vitro blood vessel model provides two channels separated by a porous membrane that is coated on one side by an endothelial cell layer and is coated on the other side by a smooth muscle cell layer, wherein said model is susceptible to the extravasation of red blood cells across said porous membrane due to drug induced vascular injury.
Owner:CHARLES STARK DRAPER LABORATORY +1
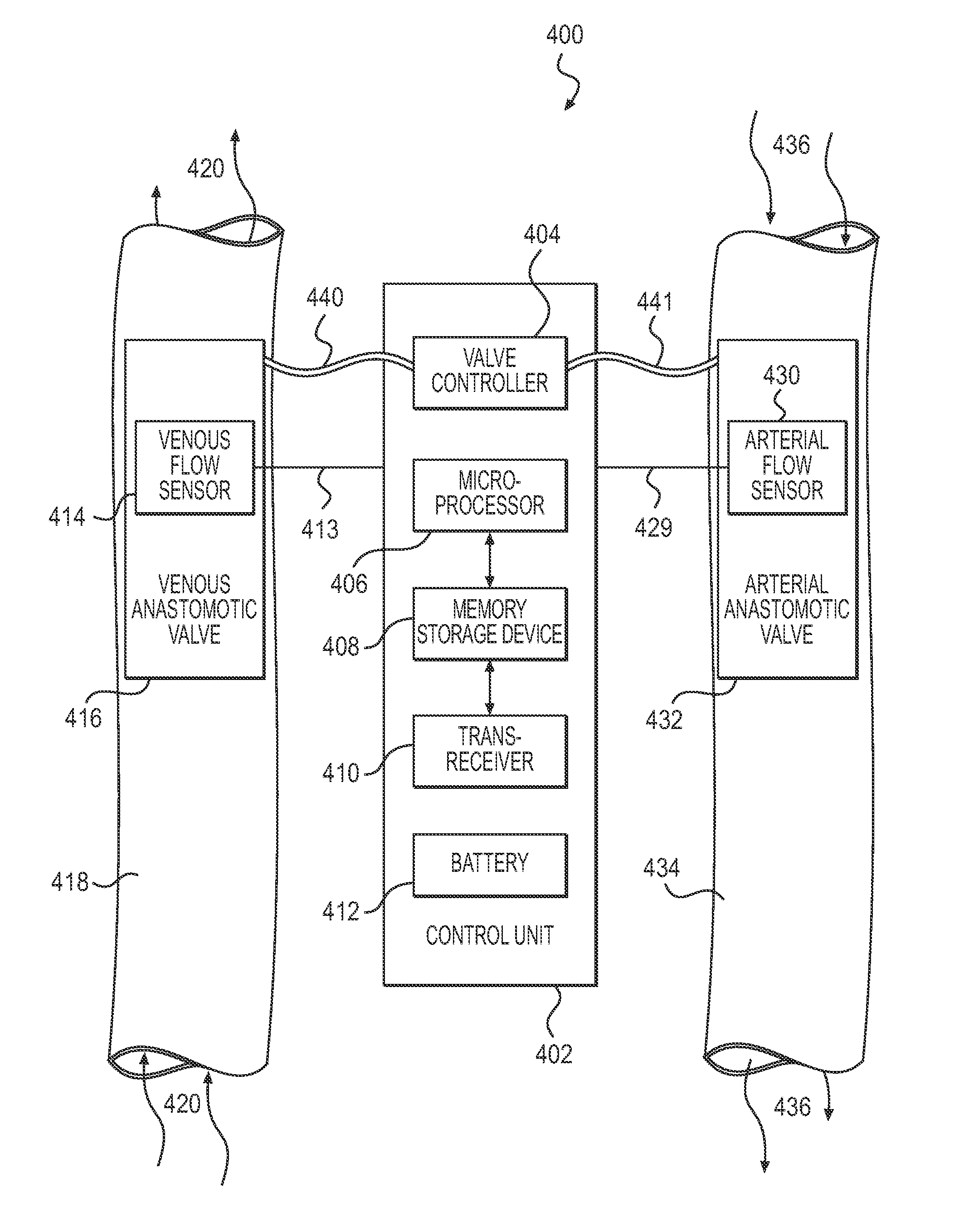
Arteriovenous shunt with integrated surveillance system
Owner:INDIANA UNIV RES & TECH CORP
Hybrid systems and methods for multi-modal acquisition of intravascular imaging data and counteracting the effects of signal absorption in blood
ActiveUS20160228097A1Blood flow measurement devicesOrgan movement/changes detectionUltrasound attenuationHybrid system
A multi-modal catheter combining at least NIRF and IVUs imaging channels used to reveal integrated biological and structural features of a lumen intravascularly imaged through flowing blood in vivo and corrected for distance-related attenuation and / or scattering parameters of in vivo blood to compensate for discovered overestimation of the degree of in-vivo-blood attenuation of NIRF signal Enhancing the sensitivity of detection of vascular injury and / or plaque deposition beyond the capability of a standalone IVUS imaging as a result of the use of such corrected catheter.
Owner:THE GENERAL HOSPITAL CORP
Device and method for inducing vascular injury and/or blockage in an animal model
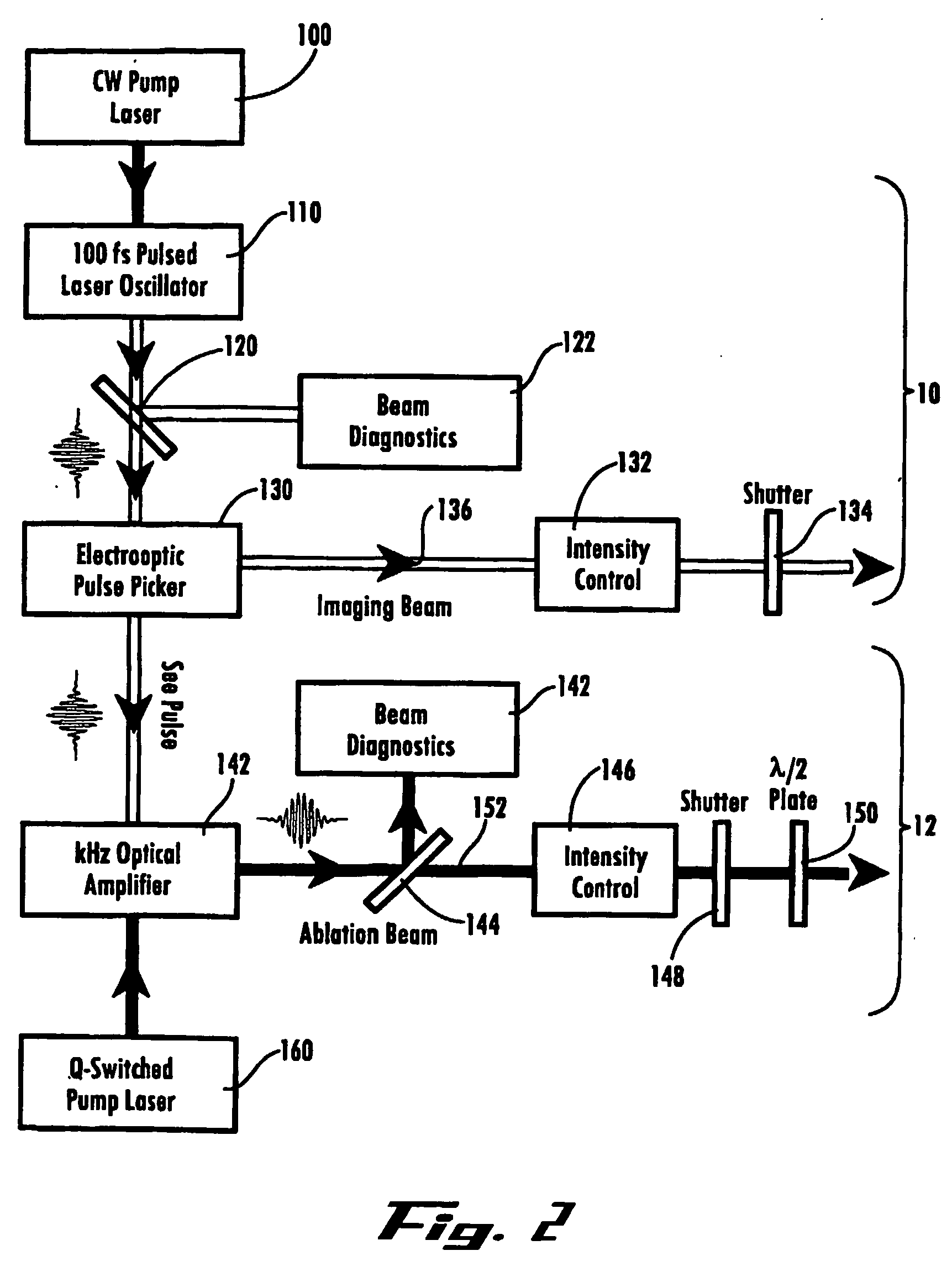
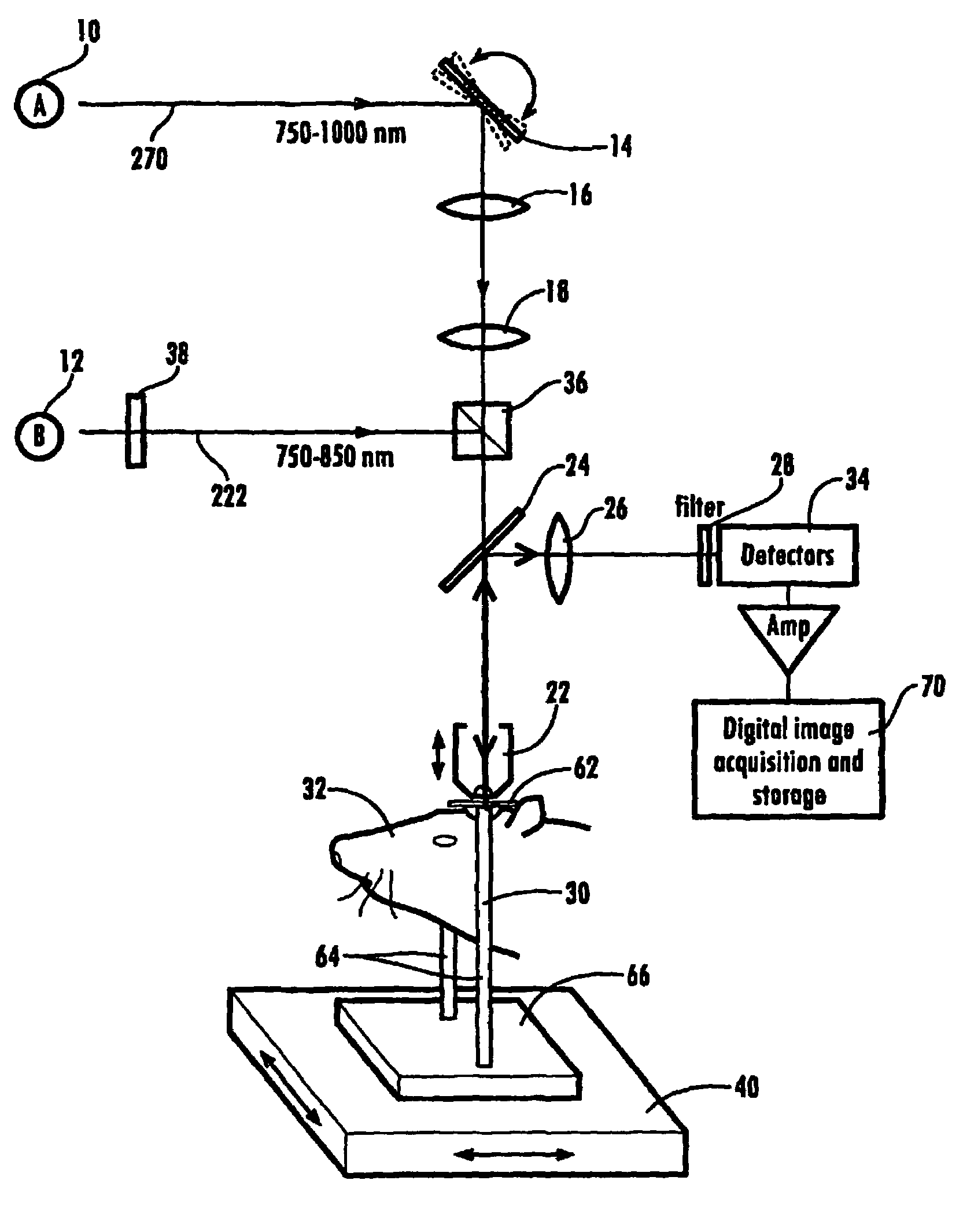
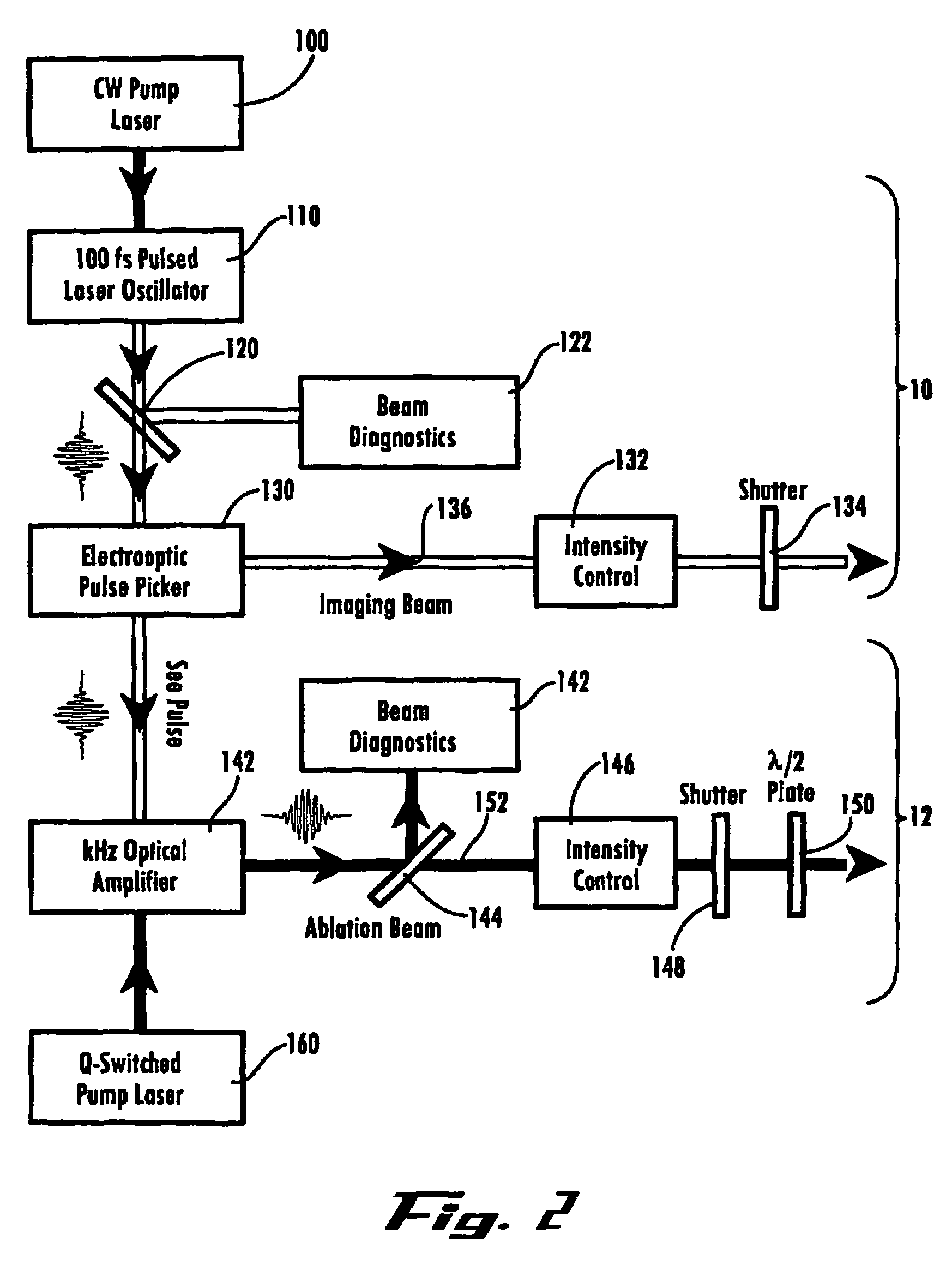
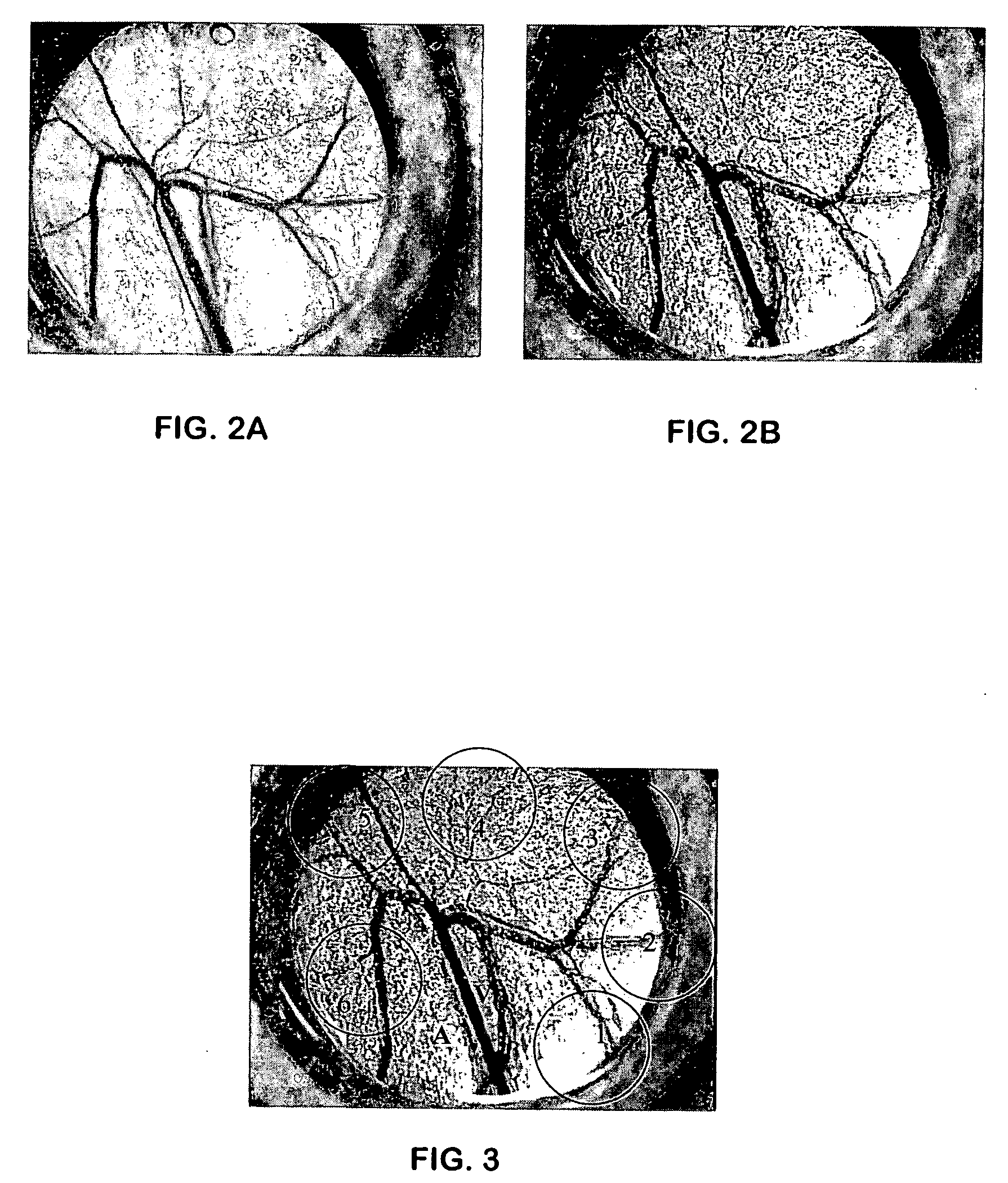
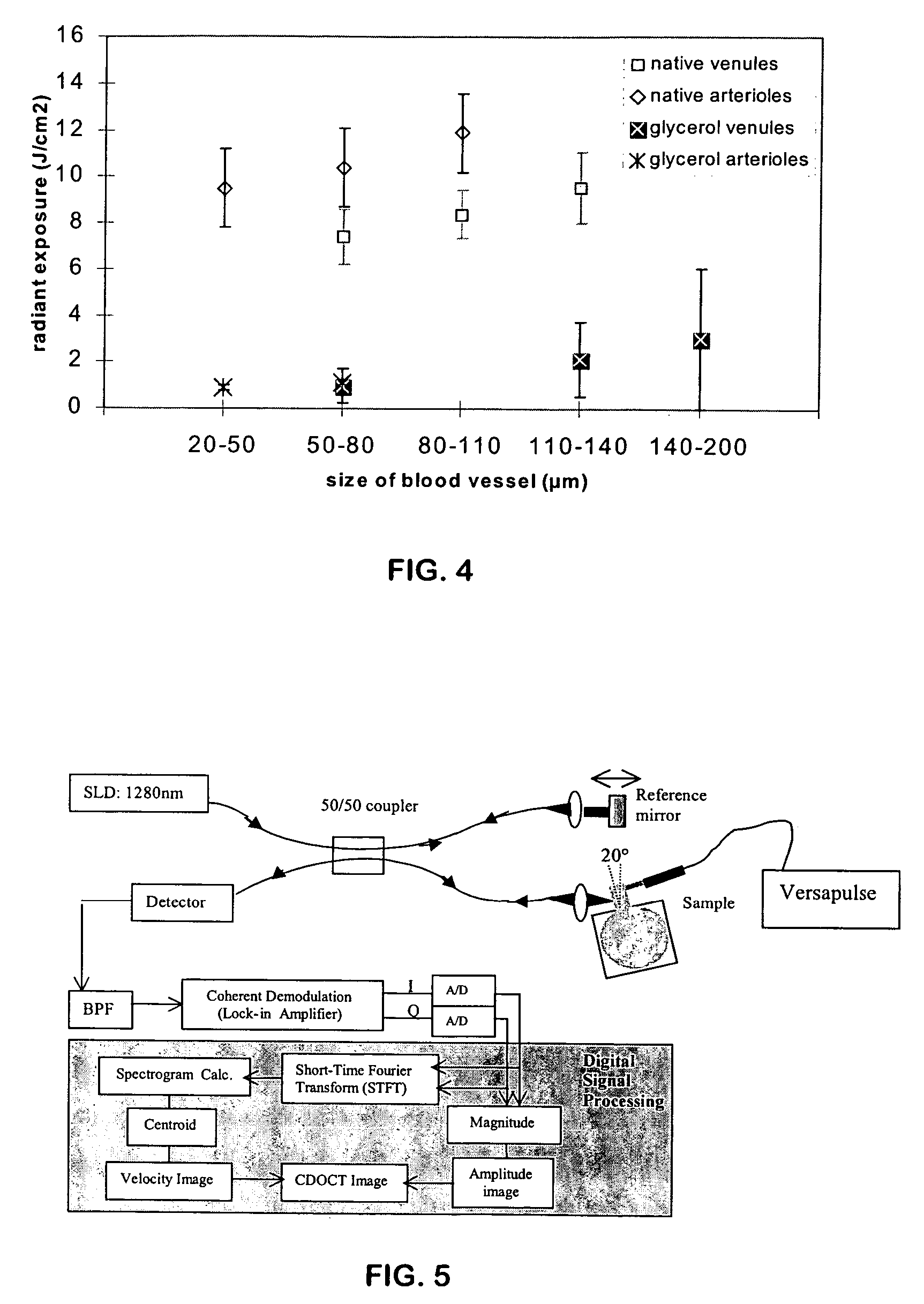
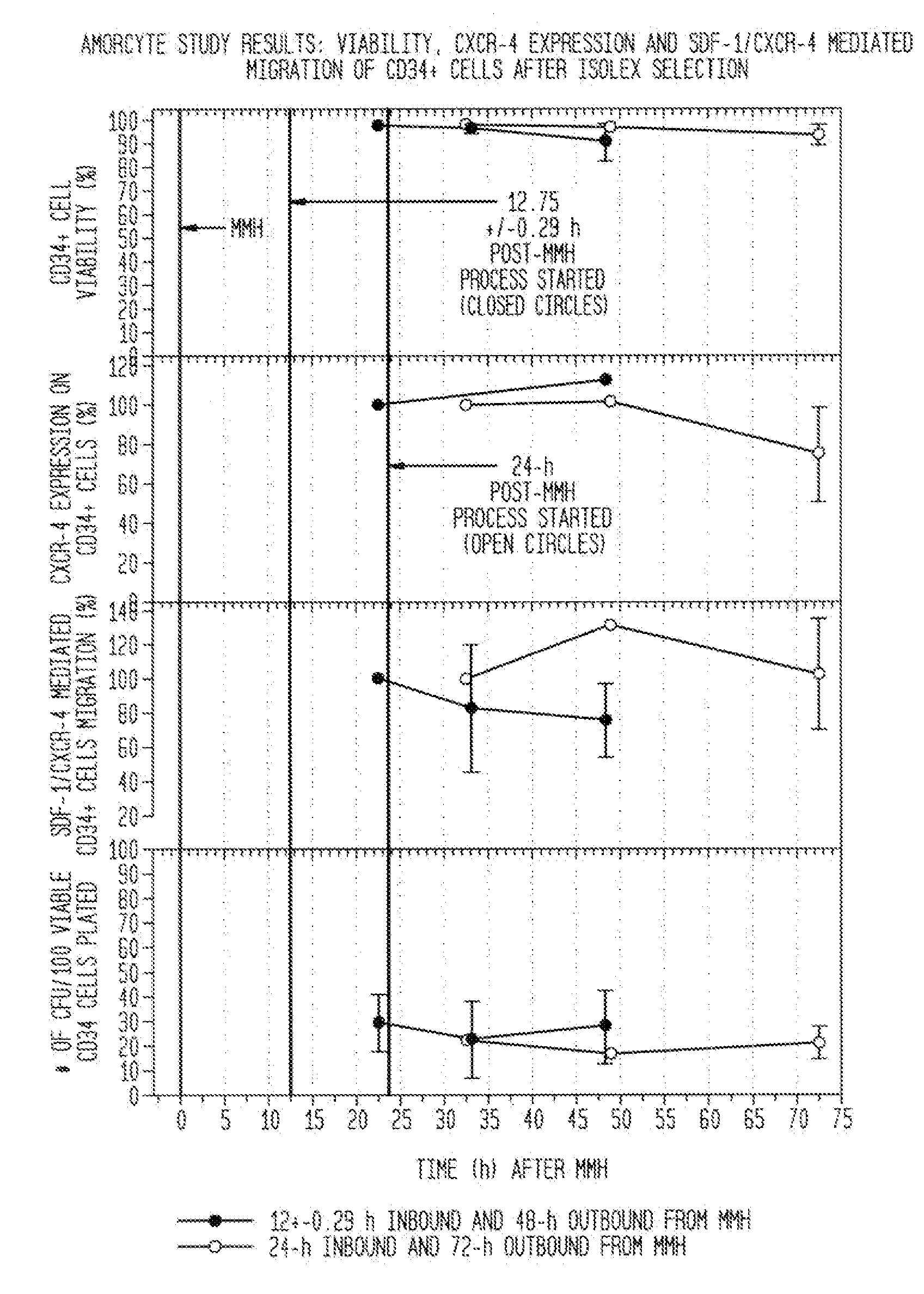
Ultrashort laser pulses are used to induce photodisruptive breakdown in vasculature in an animal to controllably produce hemorrhage, thrombosis or breach of the blood-brain barrier in individual, specifically-targeted blood vessels. Damage is limited to the targeted vessels such that neighboring vessels exhibit no signs of vascular damage, including vessels directly above and directly below the targeted vessel. Ultrashort laser pulses of lower energy are also used to observe and quantify the baseline and altered states of blood flow. Observation and measurement may be performed by TPLSM, OCT or other known techniques, providing a real-time, in vivo model for the dynamics and effects of vascular injury.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Infarct area perfusion-improving compositions and methods of vascular injury repair
ActiveUS20100143317A1Increase perfusionDecline in muscle functionBiocidePeptide/protein ingredientsBlood vessel injuryPerfusion
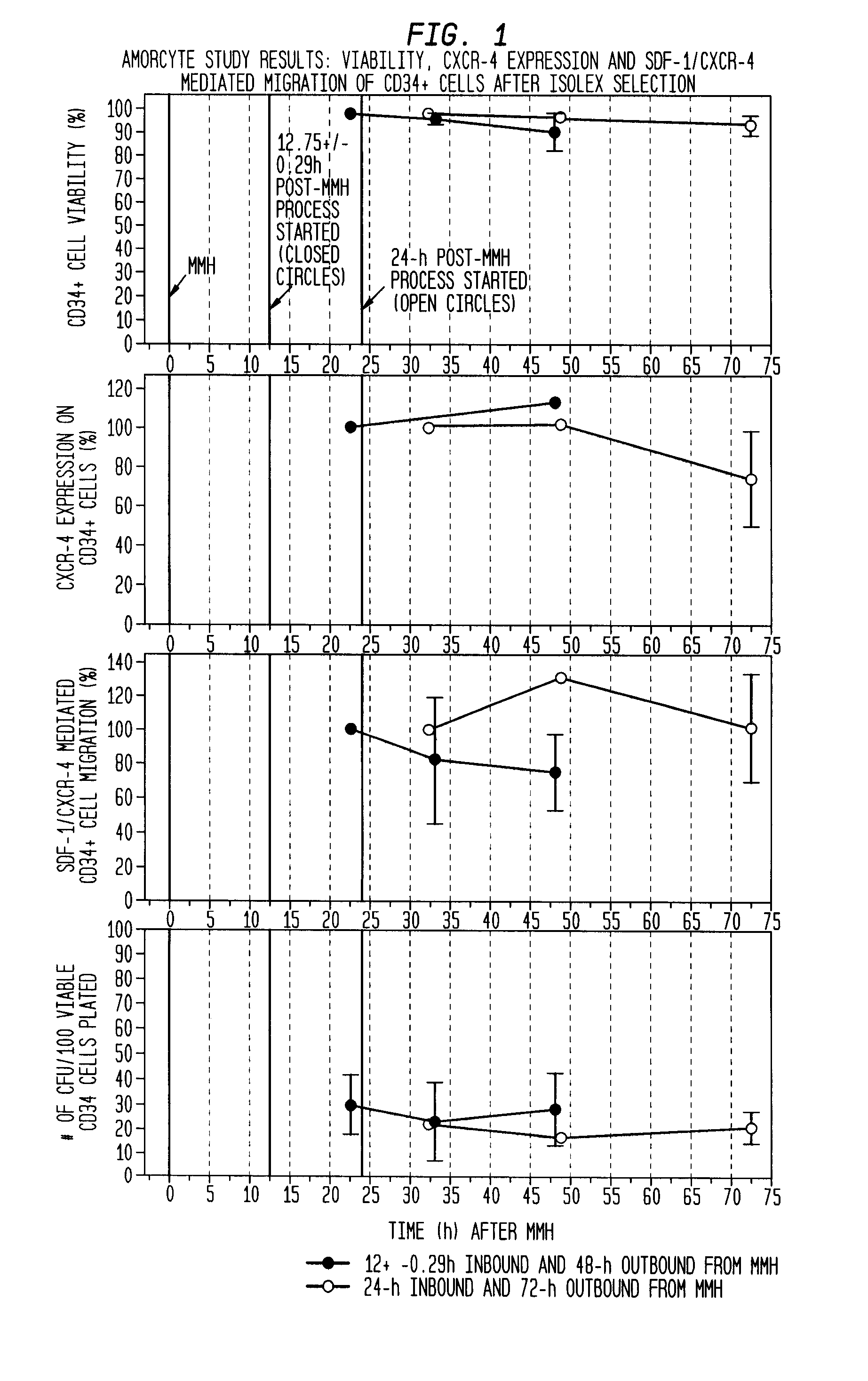
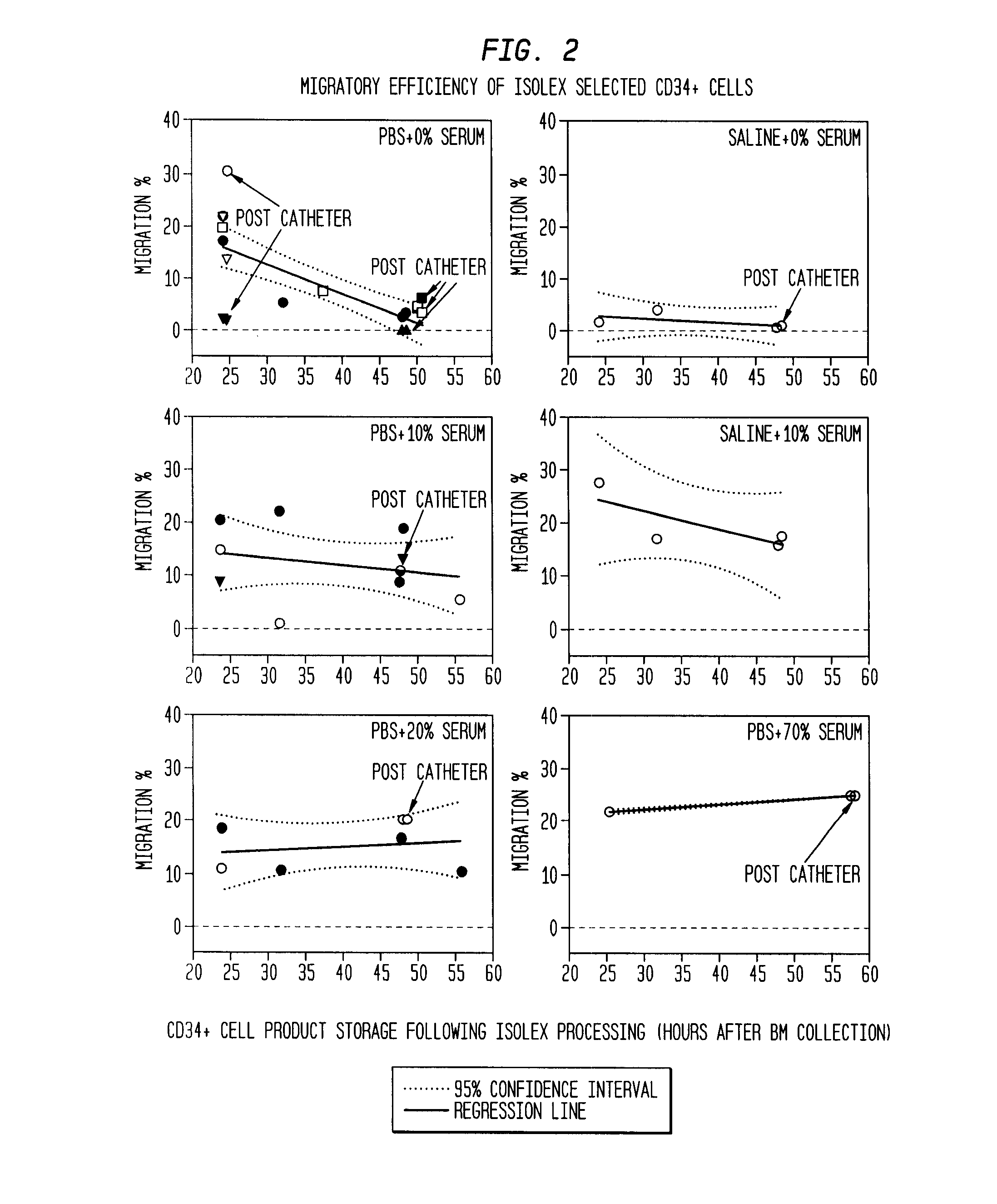
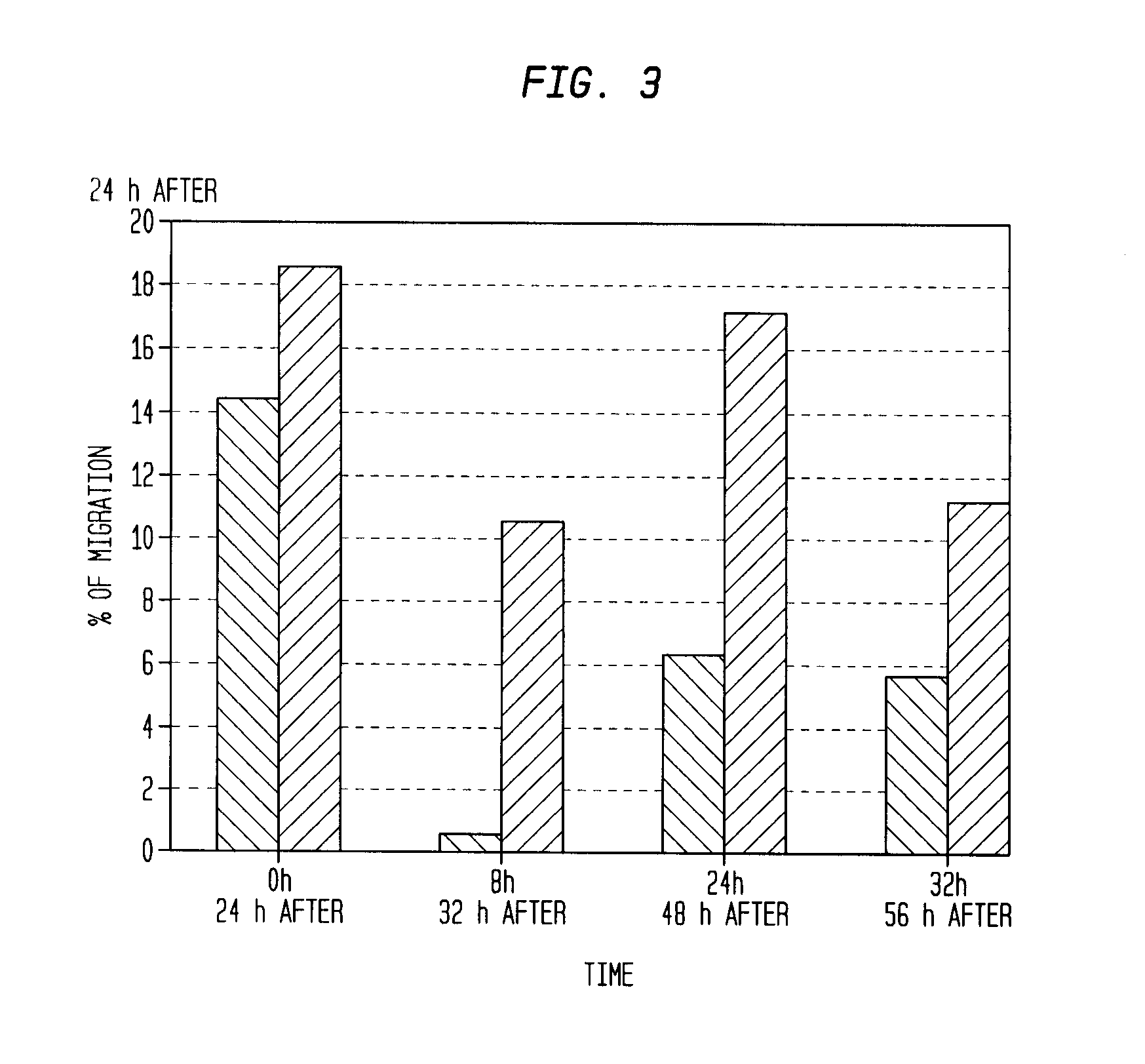
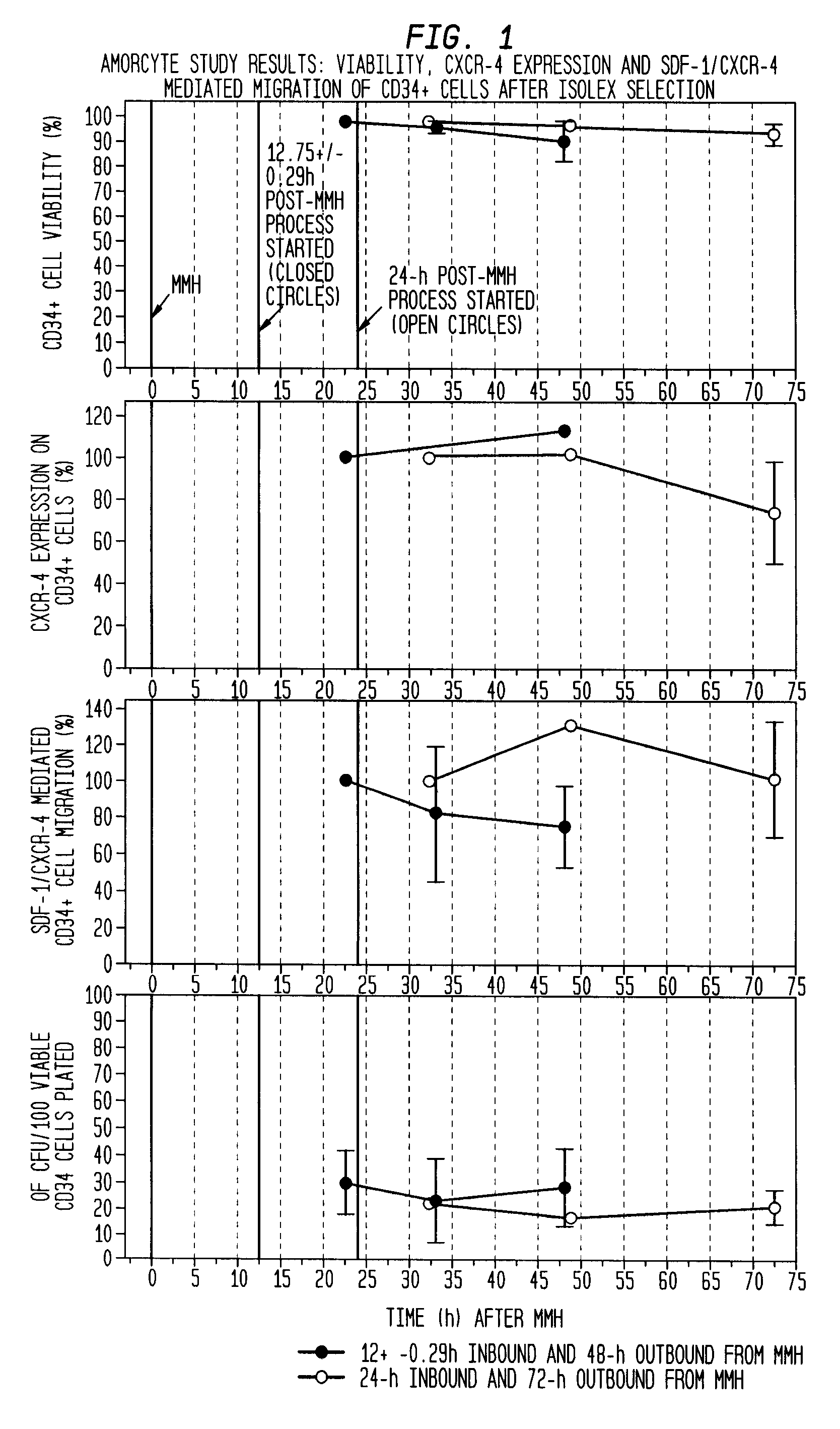
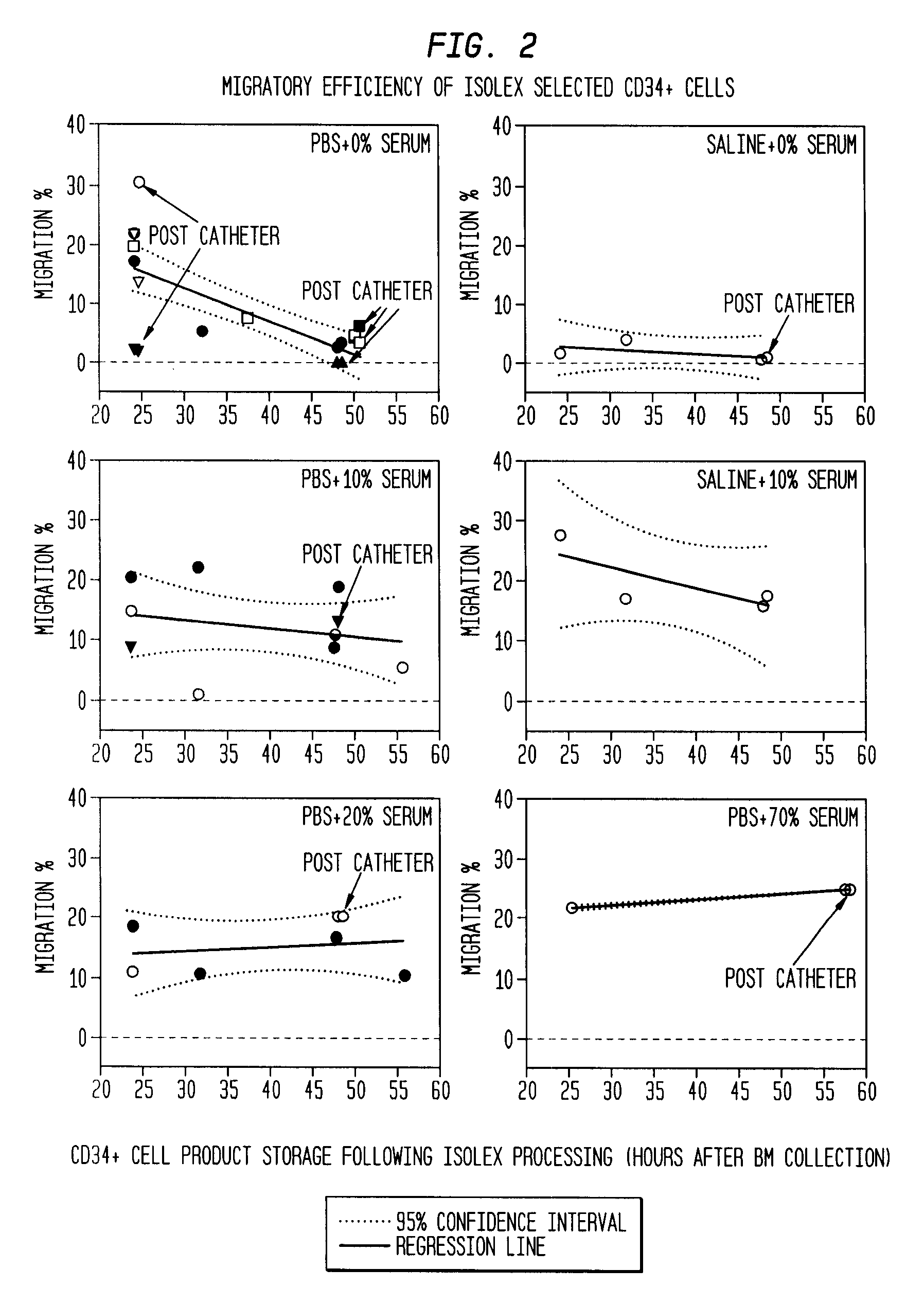
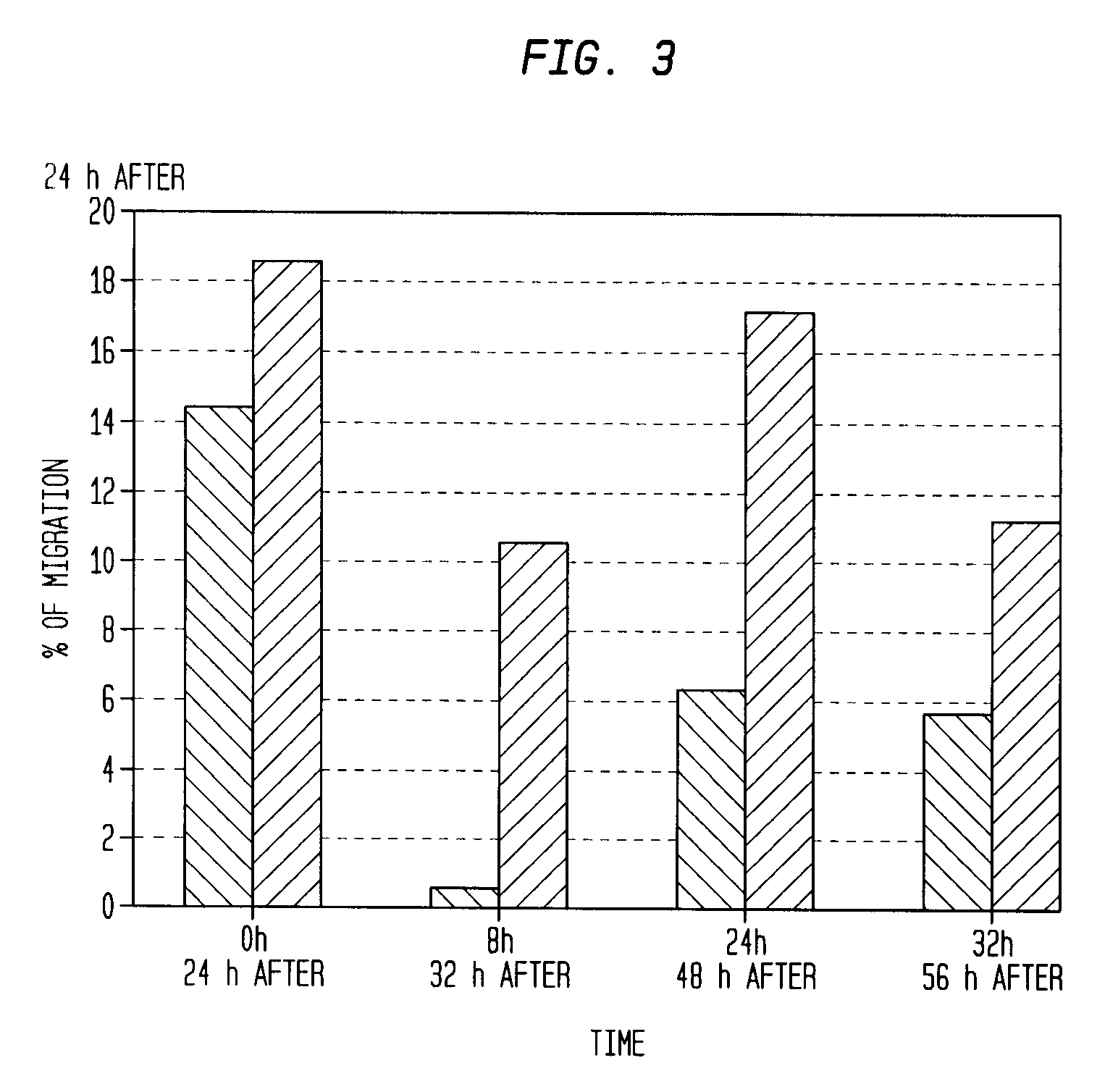
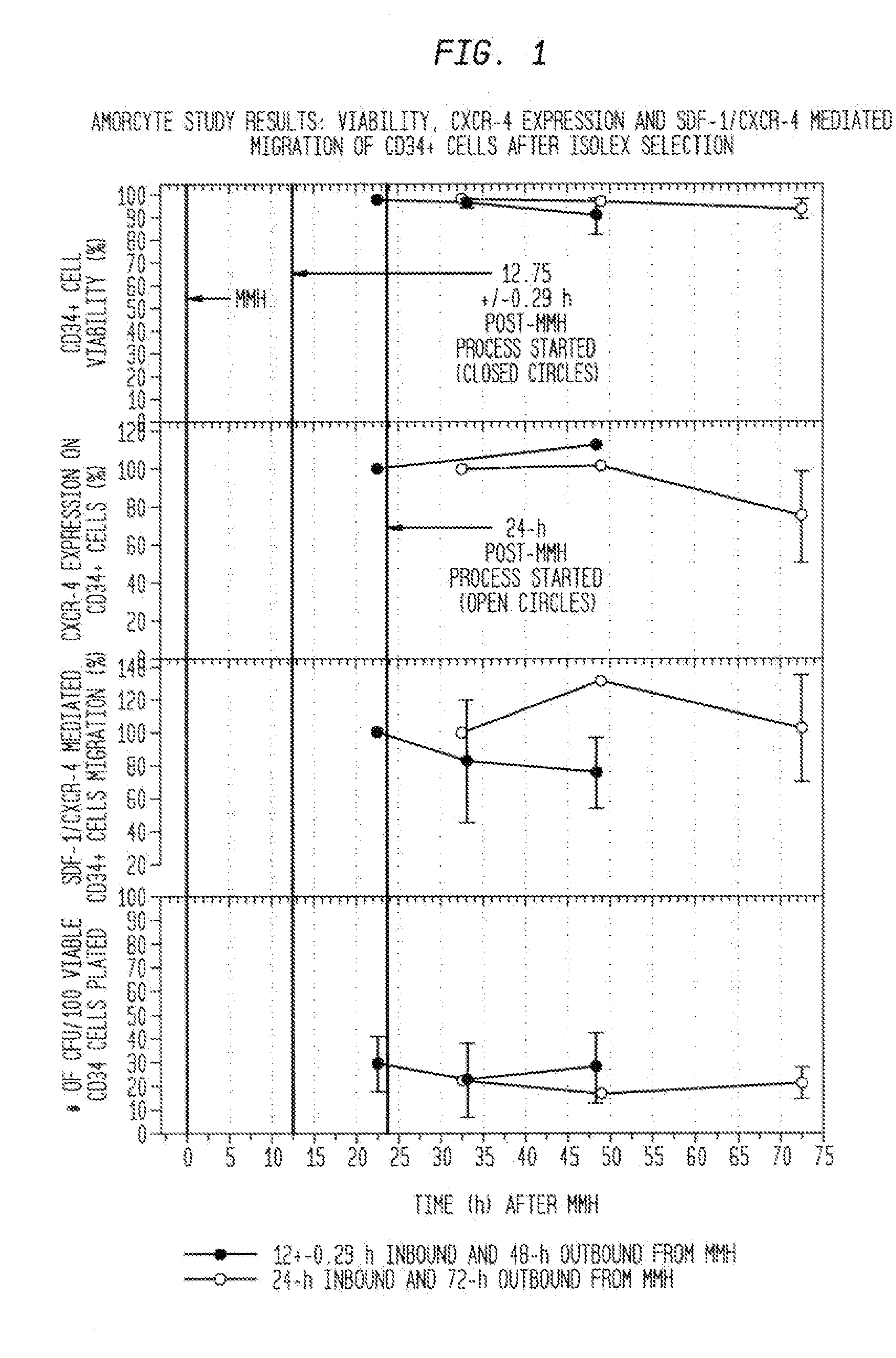
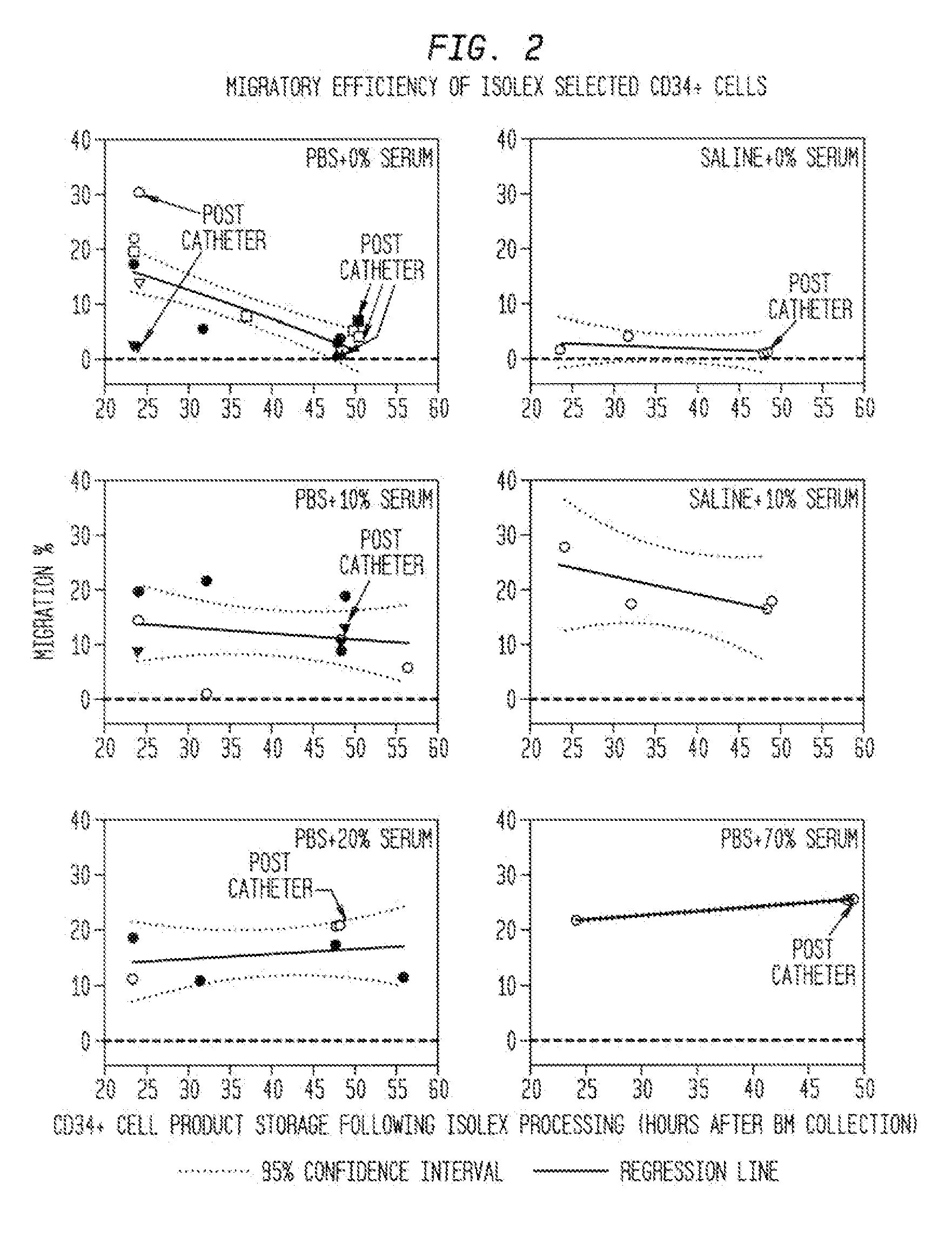
The described invention provides pharmaceutical compositions for treating an infarct area injury and methods of treating or repairing the infarct area injury in a revascularized subject in the aftermath of an acute myocardial infarction resulting from a natural disease process by administering to the subject parenterally through a catheter a sterile pharmaceutical composition containing a therapeutically effective amount of a nonexpanded sterile isolated chemotactic hematopoietic stem cell product as a first therapeutic agent and optionally a therapeutically effective amount of at least one compatible second therapeutic agent. The infarct area-improving amount of the sterile isolated chemotactic hematopoietic stem cell product comprises an enriched population of isolated autologous CD34+ cells containing a subpopulation of potent cells expressing CXCR-4 and having CXCR-4-mediated chemotactic activity such that the enriched population of isolated autologous CD34+ hematopoietic stem cells provides at least 0.5×106 potent CD34+ cells expressing CXCR-4 and having CXCR-4 mediated chemotactic activity.
Owner:CALADRIUS BIOSCI
Cutting device for blood vessel plaque
PendingCN107496009APrevent slippageReduce riskBalloon catheterMedical devicesBlood vessel spasmFibrosis
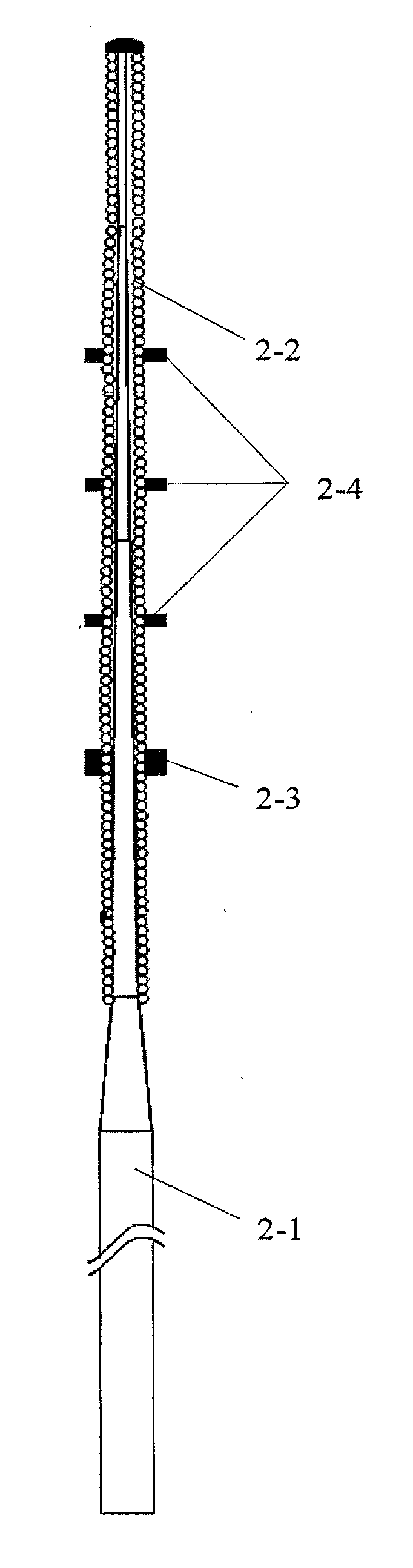
The invention discloses a cutting device for a blood vessel plaque. The cutting device comprises a plaque cutting system, a pressurized expansion system and a delivering device, wherein the cutting system comprises a plurality of cutting wires or cutting blades which are connected with a far-end cutting indicating ring and a near-end cutting indicating ring; the pressurized expansion system consists of a balloon and a balloon indicating ring; the delivering device comprises a long and thin hollow near-end pipe body connected with the pressurized expansion system, a stress transition pipe and a pressure connecting seat. The cutting device for the blood vessel plaque, disclosed by the invention, has the following characteristics that the positions of the cutting wires or the cutting blades can be seen in the cutting operation process of the blood vessel plaque, the convenience in operation is realized, and bending of the balloon is avoided; the cutting device can be used for cutting the blood vessel plaque, in particular a heavily-calcified or fibrous plaque at lower pressure without less damage to blood vessels.
Owner:GUANGDONG BROSMED MEDICAL DEVICE CO LTD
Surgical apparatus for aneurysms
InactiveUS20130066413A1Highly structuredImprove scalabilityStentsOcculdersSurgical operationBlood vessel injury
The present application discloses a surgical apparatus for aneurysms comprising: a stent, a delivery guide wire, an introducer sheath and a microcatheter, wherein: the stent is a self-expanding stent; the delivery guide wire outside of which the stent is restrained to is provided in a lumen of the introducer sheath; the introducer sheather is connected with the microcatheter, with lumnes communicating, to form a passageway through which the delivery guide wire and the stent are delivered into a human body. The surgical apparatus for aneurysms provided in the examples of the present application is able to deliver and release the stent which has high density and is super soft to a vascular lesion. A lattice structure of the stent is of high coverage at the vascular lesion such that the stent released into the vessel prodeces the same effects as healing of parent vessel, and thus improves the treatment of vascular aneurysms.
Owner:SHANGHAI MICROPORT MEDICAL (GROUP) CO LTD
Arteriovenous shunt with integrated surveillance system
ActiveUS20120059305A1Increase rate is permittedReduce rate at which blood is permittedIntravenous devicesTemperature sensorsBlood flowHematological test
A hemodialytic angioacess device for implantation in dialysis patients, comprising an arteriovenous (AV) shunt, anastomotic valves that connect the AV shunt to blood vessels, a valve control system and an integrated surveillance system that measures flow conditions in the blood vessels at the AV shunt, preferably when the valves are closed and the patient is not undergoing dialysis treatment. The flow condition data, which may include data representing the flow rate, pressure, volume and velocity of blood flowing through the vessels, is stored in a memory and transmitted to a health care provider or technician on demand. The data can be used to calculate and monitor important physiological parameters, such as compliance and resistance, for the blood vessels, and help detect and identify dangerous conditions, such as turbulence and stasis, which can contribute to AV shunt failure, vessel injury and other serious complications for the patient.
Owner:INDIANA UNIV RES & TECH CORP
Preparation of medical bioavailability bracket material and uses thereof
The invention relates to a preparation and using method of medical biocompatible stent material. The medical biocompatible stent material is prepared by applying the small intestine submucosal and the tendon tissue of people or animals as the raw materials. The invention has good biocompatibility, appropriate porosity and mechanical strength and stable spatial structure, and the invention contains various growth factors to induce the tissue for regeneration and reconstruction, thereby improving the success rate of the repair of the various tissue defects and the reconstruction of tissue structures, therefore, the invention is an ideal tissue engineering material. The medical biocompatible stent material which is provided by the invention is applicable to the preparation of various tissue engineering equipments and the application in the repair of various tissue defects and injuries, including skin, blood vessels, nerves, tendons, bones, cartilages, abdominal walls, urinary tracts, oral mucosa and so on, and the invention can be used in the treatment of trauma, abdominal external hernia, anal fistula, rectovaginal fistula, rectum anterior protrusion, bone and cartilage defects, vascular injuries, nerve injuries, tendon injuries, oral mucosal injuries, relaxed pelvic floor, urinary tract injuries and other diseases.
Owner:BEIJING BIOSIS HEALING BIOLOGICAL TECH
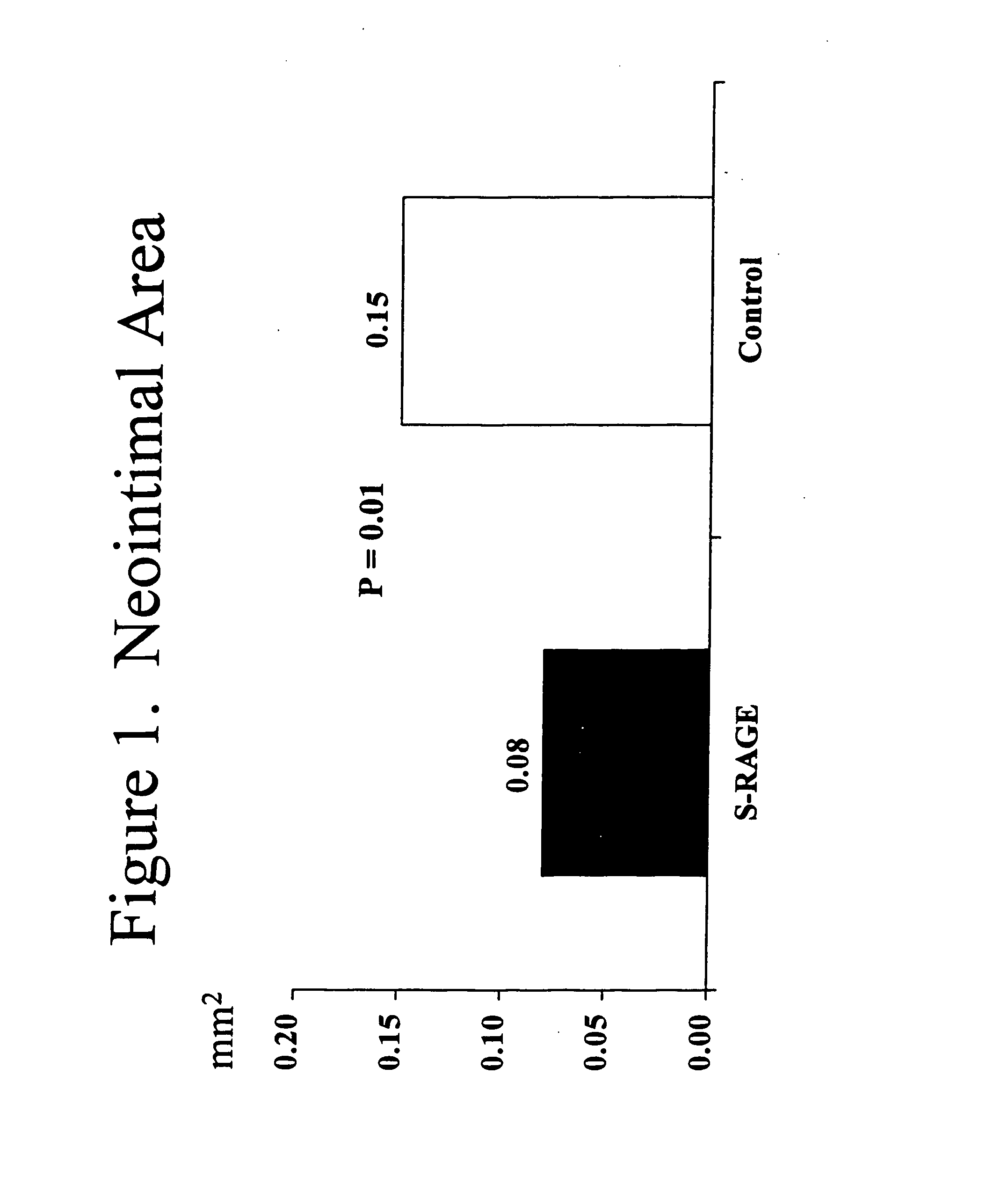
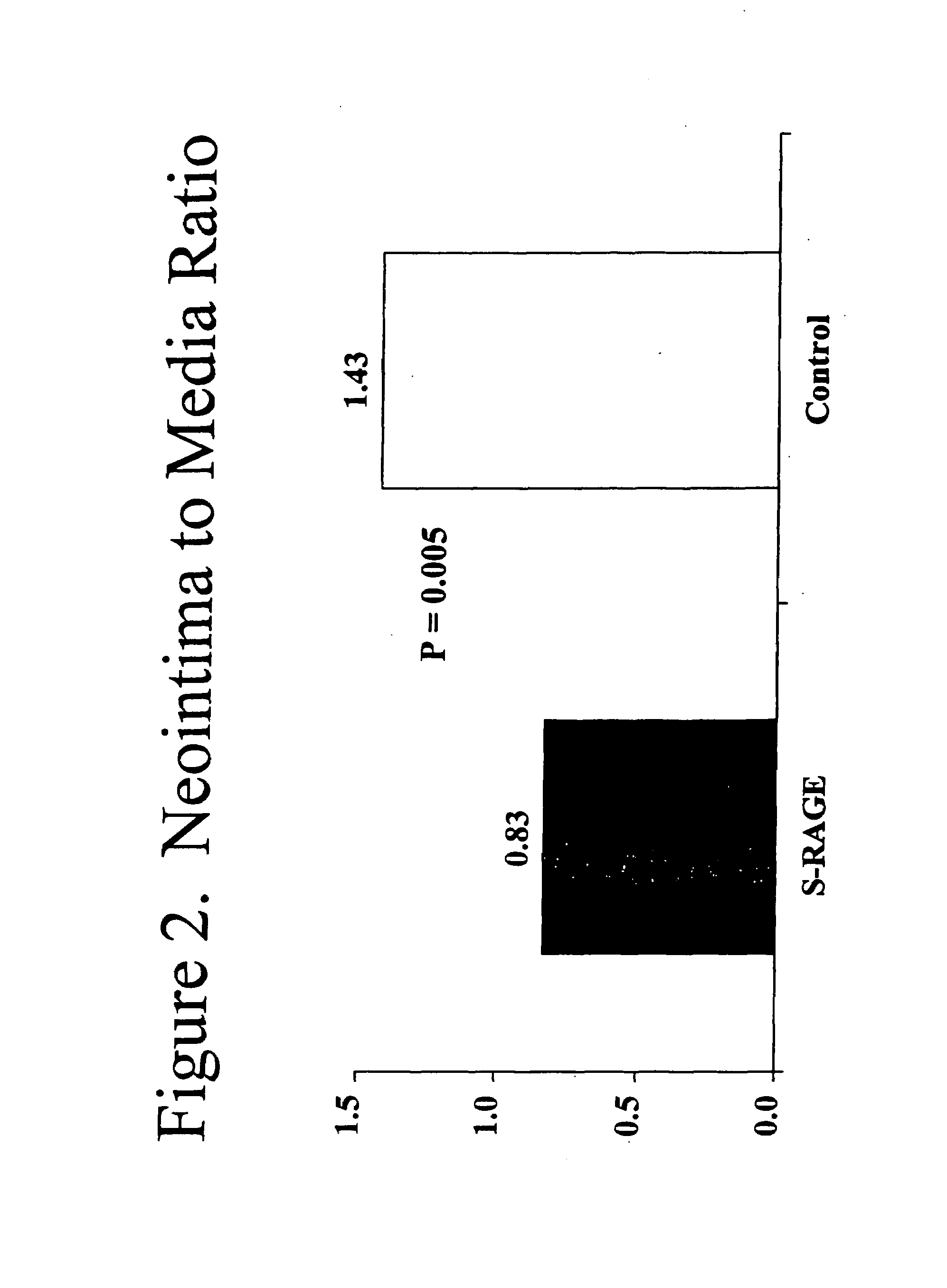
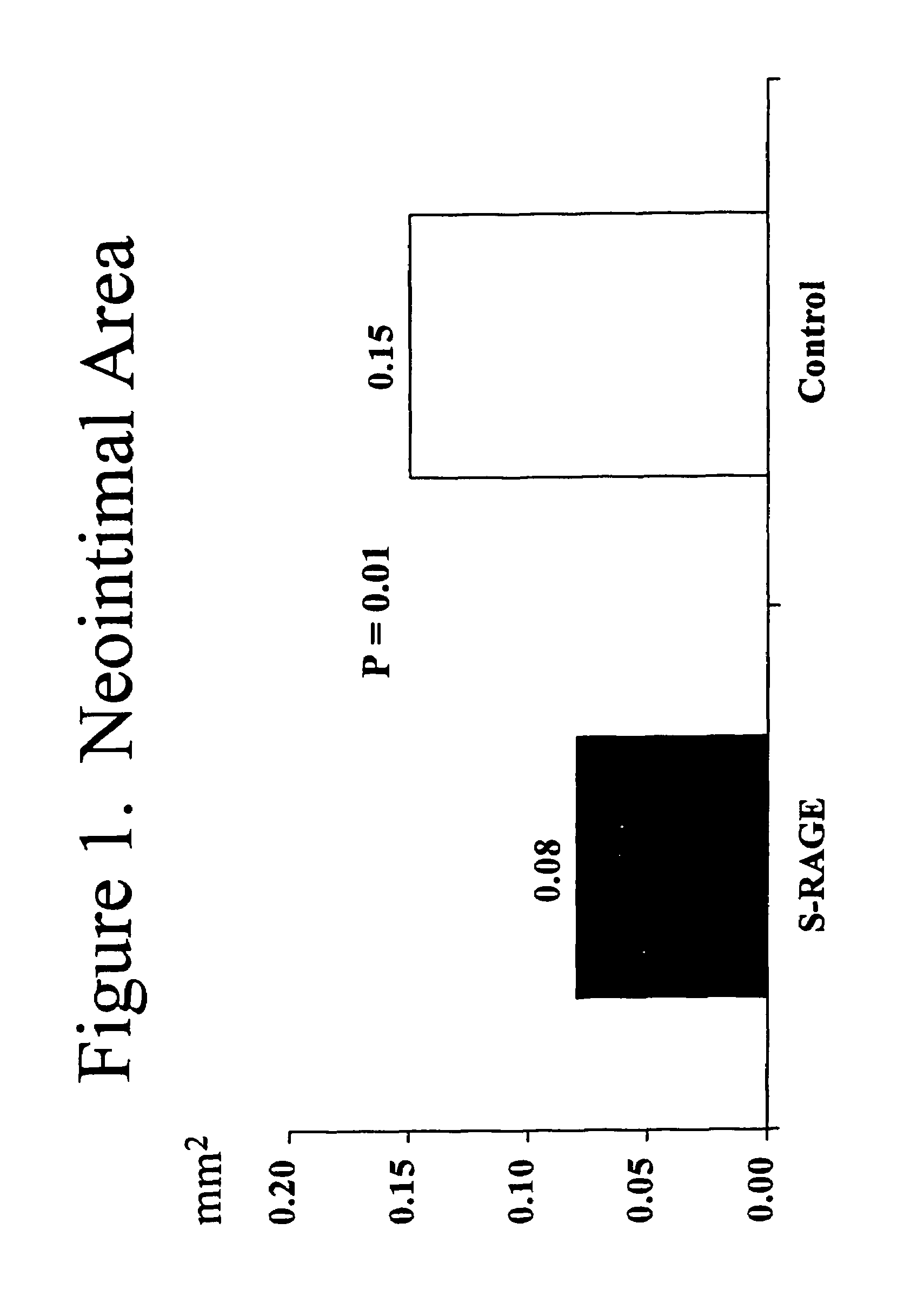
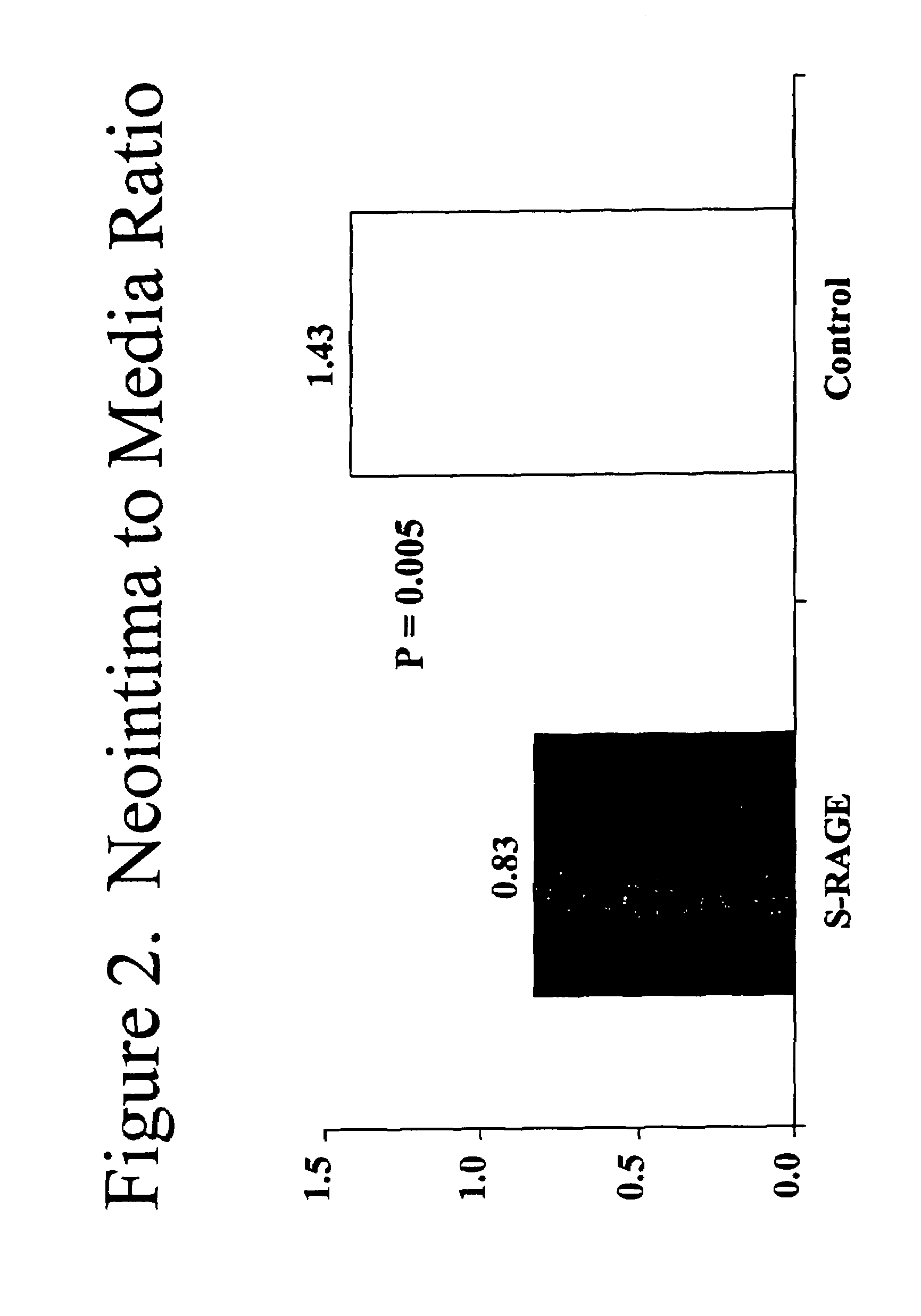
Method for inhibiting new tissue growth in blood vessels in a patient subjected to blood vessel injury
InactiveUS20080171701A1Preventing exaggerated restenosisPrevent exaggerated restenosisOrganic active ingredientsPeptide/protein ingredientsBlood vessel spasmPercent Diameter Stenosis
This invention provides for a method for inhibiting new tissue growth in blood vessels in a subject, wherein the subject experienced blood vessel injury, which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to inhibit new tissue growth in the subject's blood vessels. The invention also provides for method for inhibiting neointimal formation in blood vessels in a subject, wherein the subject experienced blood vessel injury, which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to inhibit neointimal formation in the subject's blood vessels. The invention also provides a method for preventing exaggerated restenosis in a diabetic subject which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to prevent exaggerated restenosis in the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Drug-delivery endovascular stent and method for treating restenosis
An intravascular stent and method for inhibiting restenosis, following vascular injury, is disclosed. The stent has an expandable, linked-filament body and a drug-release coating formed on the stent-body filaments, for contacting the vessel injury site when the stent is placed in-situ in an expanded condition. The coating releases, for a period of at least 4 weeks, a restenosis-inhibiting amount of the macrocyclic triene immunosuppressive compound everolimus. The stent, when used to treat a vascular injury, gives good protection against clinical restenosis, even when the extent of vascular injury involves vessel overstretching by more than 30% diameter. Also disclosed is a stent having a drug-release coating composed of (i) 10 and 60 weight percent poly-dl-lactide polymer substrate and (ii) 40–90 weight percent of an anti-restenosis compound, and a polymer undercoat having a thickness of between 1–5 microns.
Owner:BIOSENSORS INT GROUP
Method for inhibiting new tissue growth in blood vessels in a patient subjected to blood vessel injury
InactiveUS7732400B2Preventing exaggerated restenosisPrevent exaggerated restenosisBiocideOrganic active ingredientsDiabetes mellitusPercent Diameter Stenosis
This invention provides for a method for inhibiting new tissue growth in blood vessels in a subject, wherein the subject experienced blood vessel injury, which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to inhibit new tissue growth in the subject's blood vessels. The invention also provides for method for inhibiting neointimal formation in blood vessels in a subject, wherein the subject experienced blood vessel injury, which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to inhibit neointimal formation in the subject's blood vessels. The invention also provides a method for preventing exaggerated restenosis in a diabetic subject which comprises administering to the subject a pharmaceutically effective amount of an inhibitor of receptor for advanced glycation endproduct (RAGE) so as to prevent exaggerated restenosis in the subject.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
Percutaneous vascular injury treatment systems and methods
InactiveUS20180214160A1Fast, effective, non-occlusive bleeding controlReduce morbidityTourniquetsTibiaHaemodialysis machine
The present invention relates to a compressive band and hemostatic dressing system and related methods of use to provide rapid, non-occlusive bleeding control, arterial injury treatment, and injury protection comfortably and reliably after vascular access procedures. The present invention may comprise some or all of the following elements: a positioner assembly, a compressive cushion component, a compression plate, a strap with a flexible hinge assembly, and a hemostatic dressing. In a preferred embodiment, the systems and methods are applied to treat and ameliorate vascular injury in a transradial percutaneous access procedure and protect against underlying vascular injury; however, the systems and methods described herein may be applied to any vascular access procedure including arm or leg vascular approaches such as the femoral, brachial, dorsalis pedis or tibial blood vessels in arterial or venous line or sheath removal or trocar removal, and in hemodialysis.
Owner:TRICOL BIOMEDICAL INC
Methods to ameliorate and image angioplasty-induced vascular injury
InactiveUS20070140965A1Maximize distributionReduce restenosis and thrombosisPowder deliveryOrganic active ingredientsEmulsionMedicine
Methods for inhibiting restenosis in blood vessels expanded by angioplasty are described. The method comprises administering blood vessel wall-targeted emulsion containing an anti-restenotic agent.
Owner:BARNES JEWISH HOSPITAL
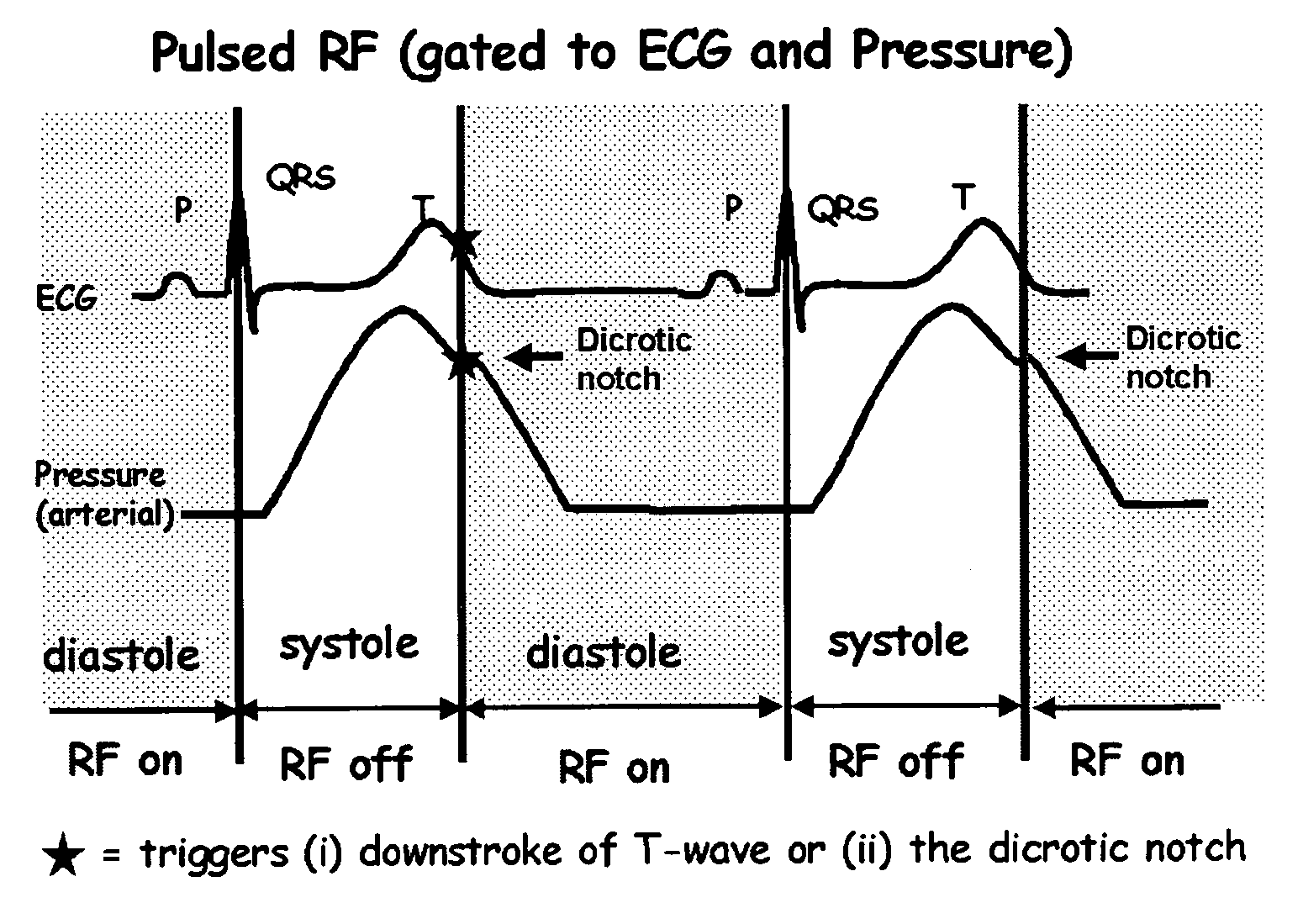
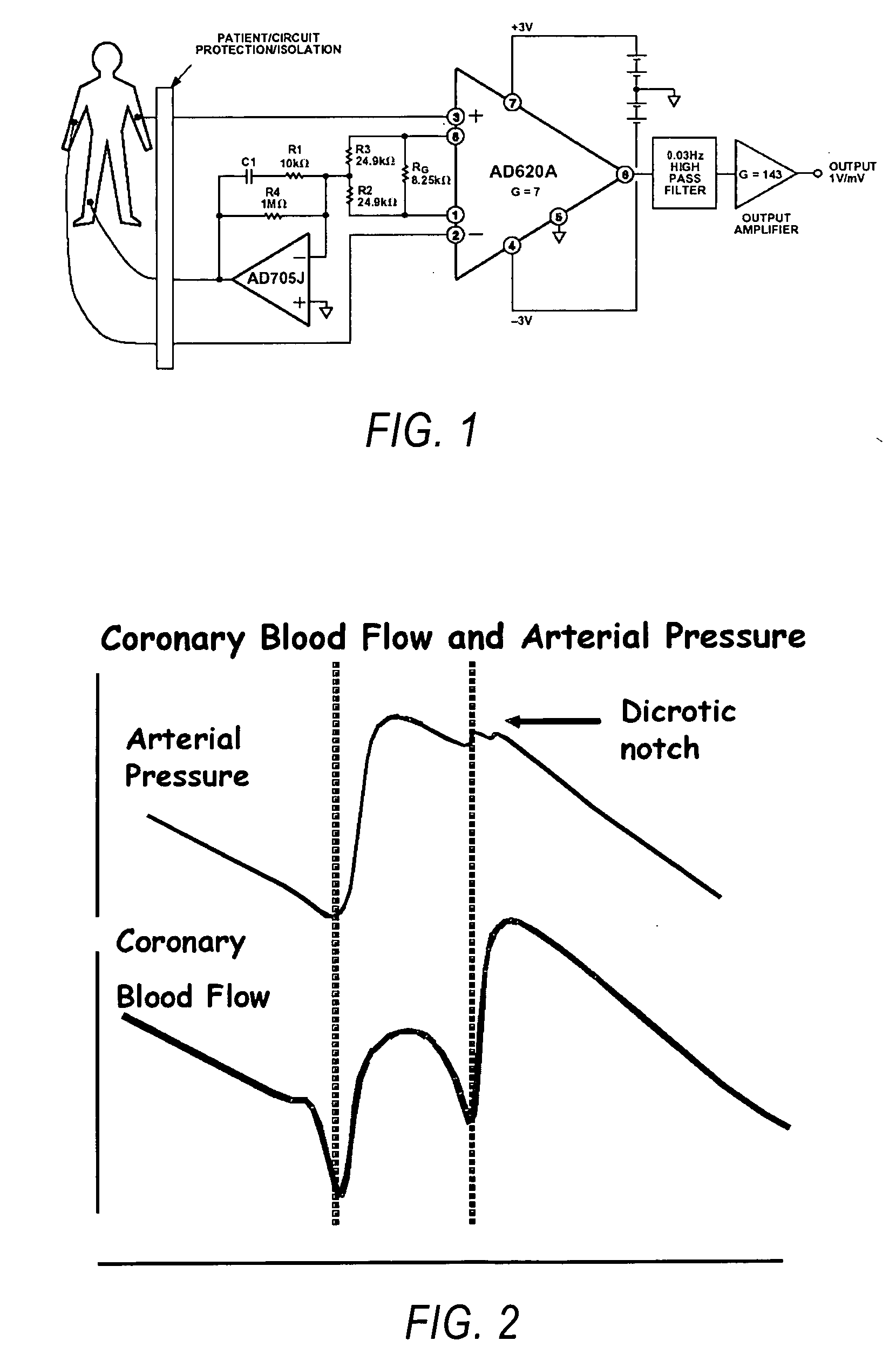
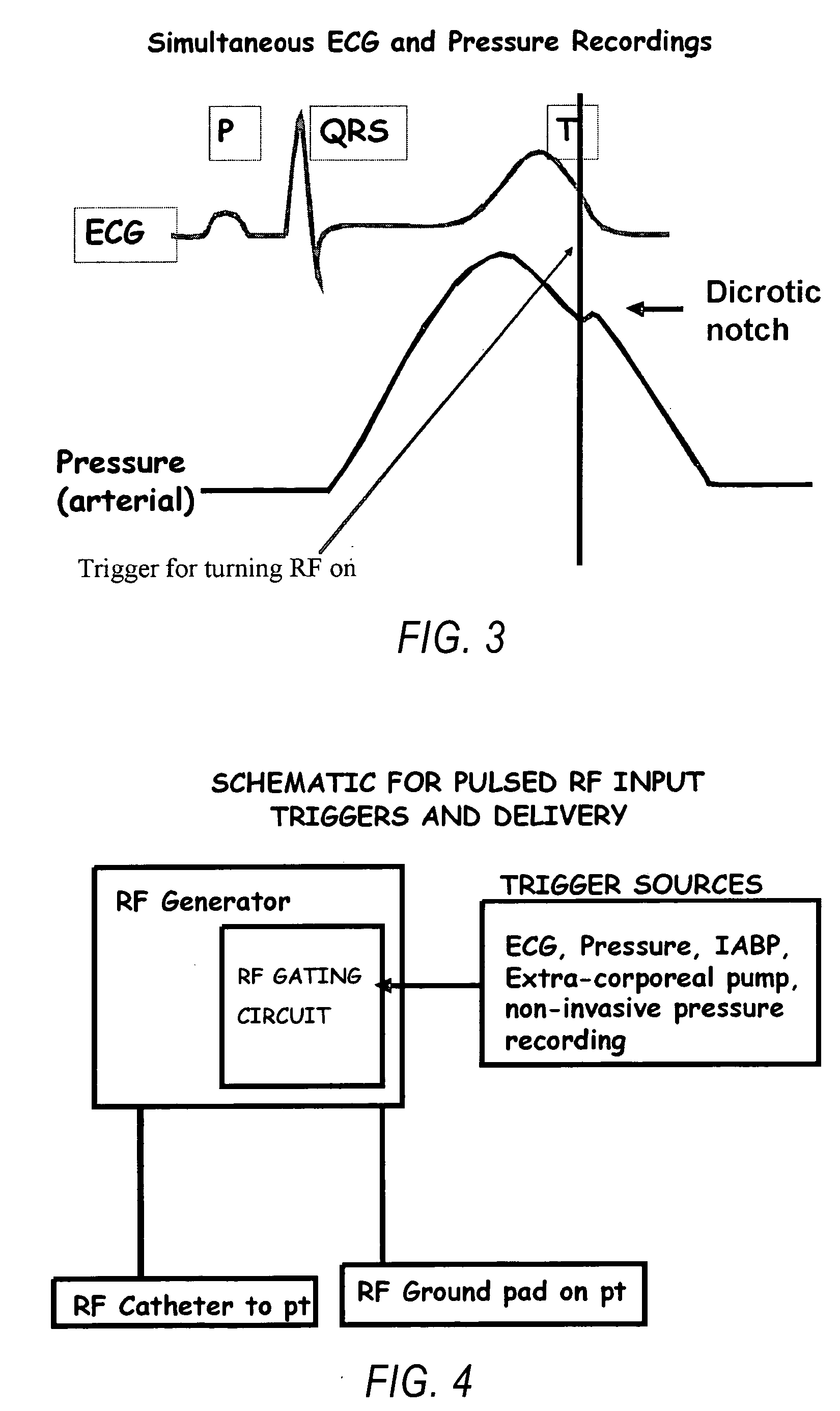
Methods and systems for gated or pulsed application of ablative energy in the treatment of cardiac disorders
InactiveUS20060200118A1Rapid coronary blood flowMinimize damageSurgical instruments for heatingDiseasePressure curve
The present invention comprises methods and systems for treating a cardiac arrhythmia in a mammal by administering gated or pulsed radiofrequency current or other ablative energy to the mammal during one or more time periods of increased coronary blood flow. Preferred embodiments of the invention comprise, without limitation, the gated or pulsed administration of radiofrequency current in association with the formation of the dicrotic notch in the arterial blood pressure curve of the mammal. In accordance with the invention, the thermal effects of ablative energy application on the coronary artery are avoided or mitigated due to rapid coronary blood flow resulting in heat loss and minimization of damage to blood vessels.
Owner:HENRY FORD HEALTH SYST
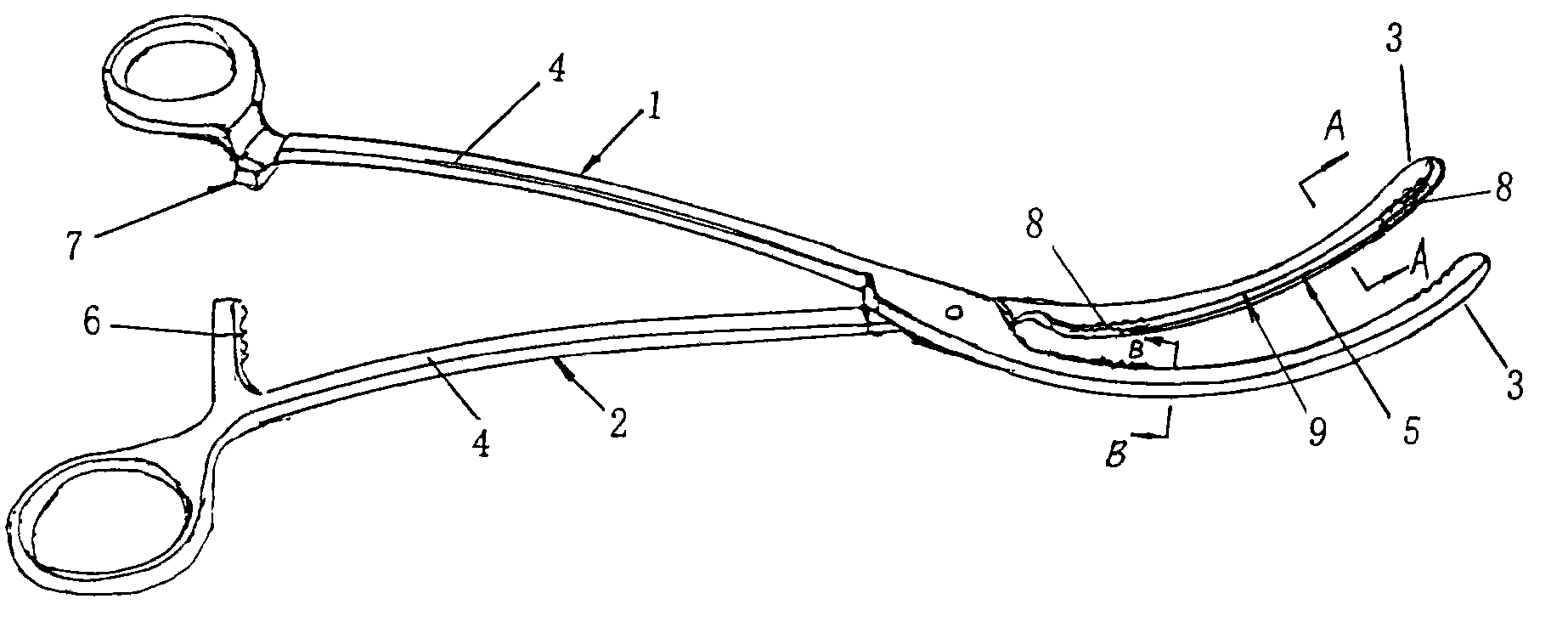
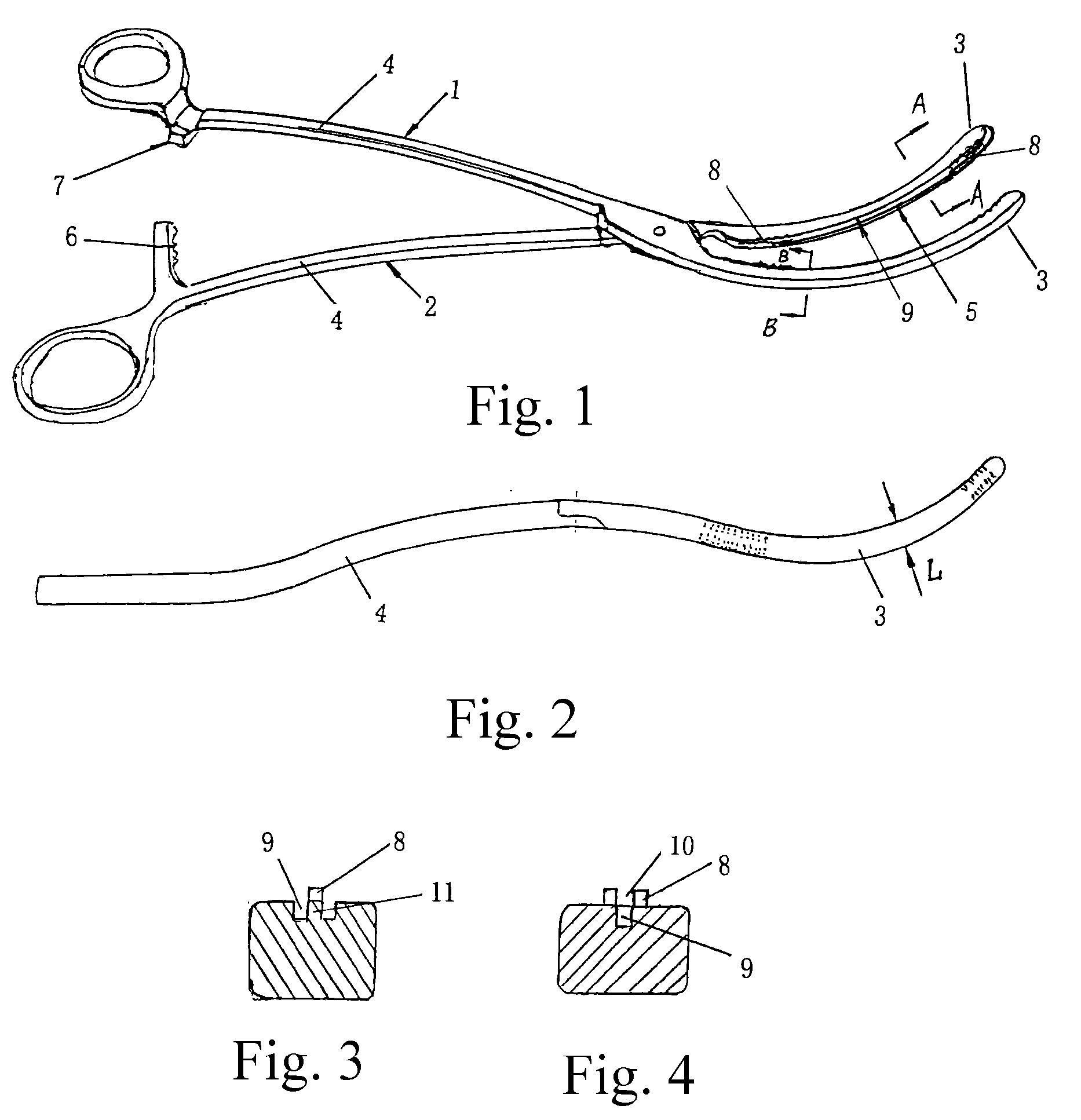
Atraumatic Hemostatic Clamp
An atraumatic hemostatic clamp, being an artery / vein clamp in the field of medical instruments, mainly includes a left clamp body (1) and a right clamp body (2) which are joined by a hinge and may be divided into jaws (3) and handles (4) by the hinge. The jaws (3) have a curved configuration with ends extending upwardly. Serrated portions (8) with serrations projecting from engaging surfaces are formed on both ends of each of the jaws (3) of the left and right clamp bodies (1), (2). A groove (9) is formed between the serrated portions (8) on both ends. Therefore, this atraumatic hemostatic clamp has a simple structure and can provide a reliable grasp without causing trauma to bodily vessels, thus solving the problem of the conventional hemostatic clamp that vessel trauma may be easily caused.
Owner:XU LIN
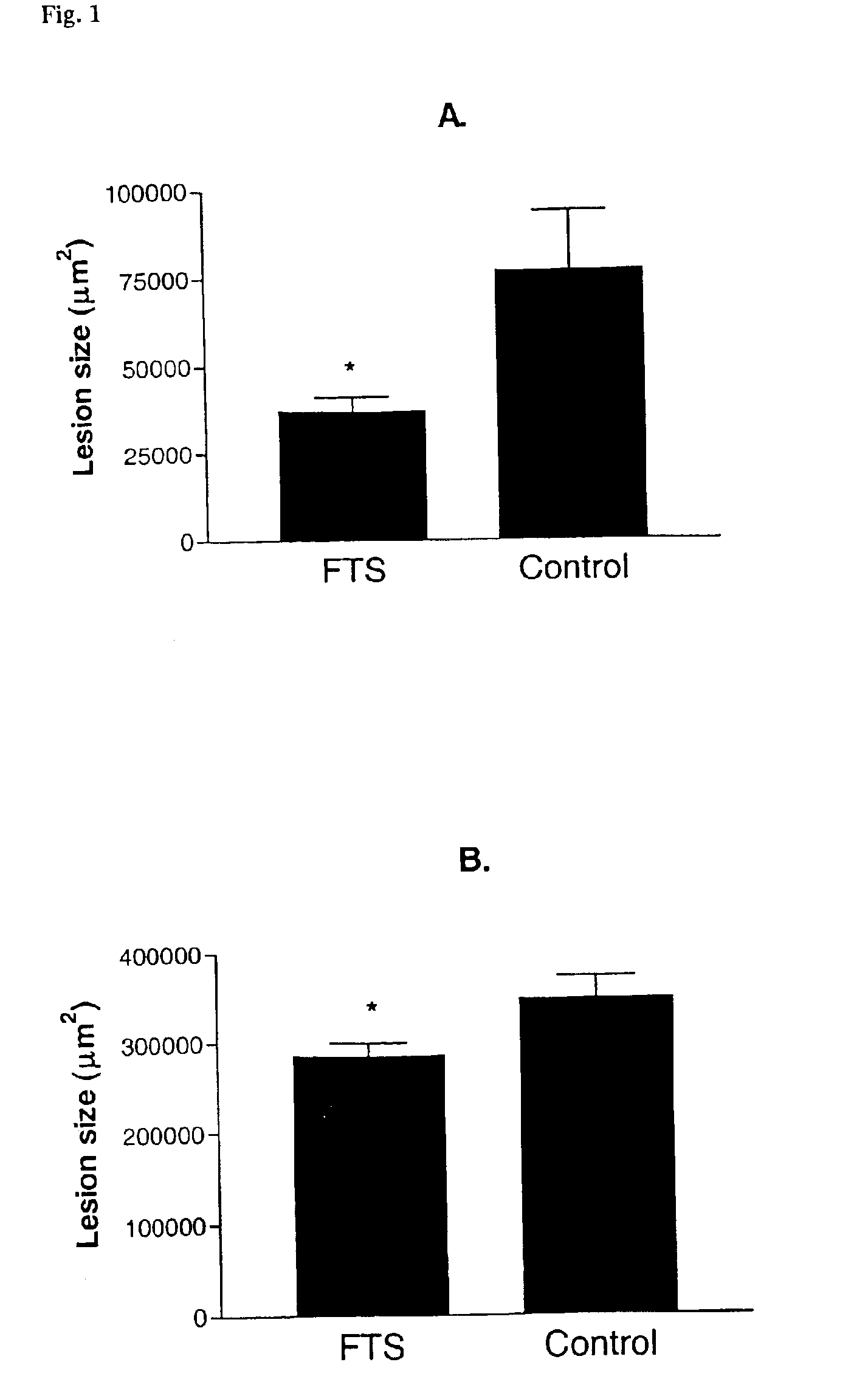
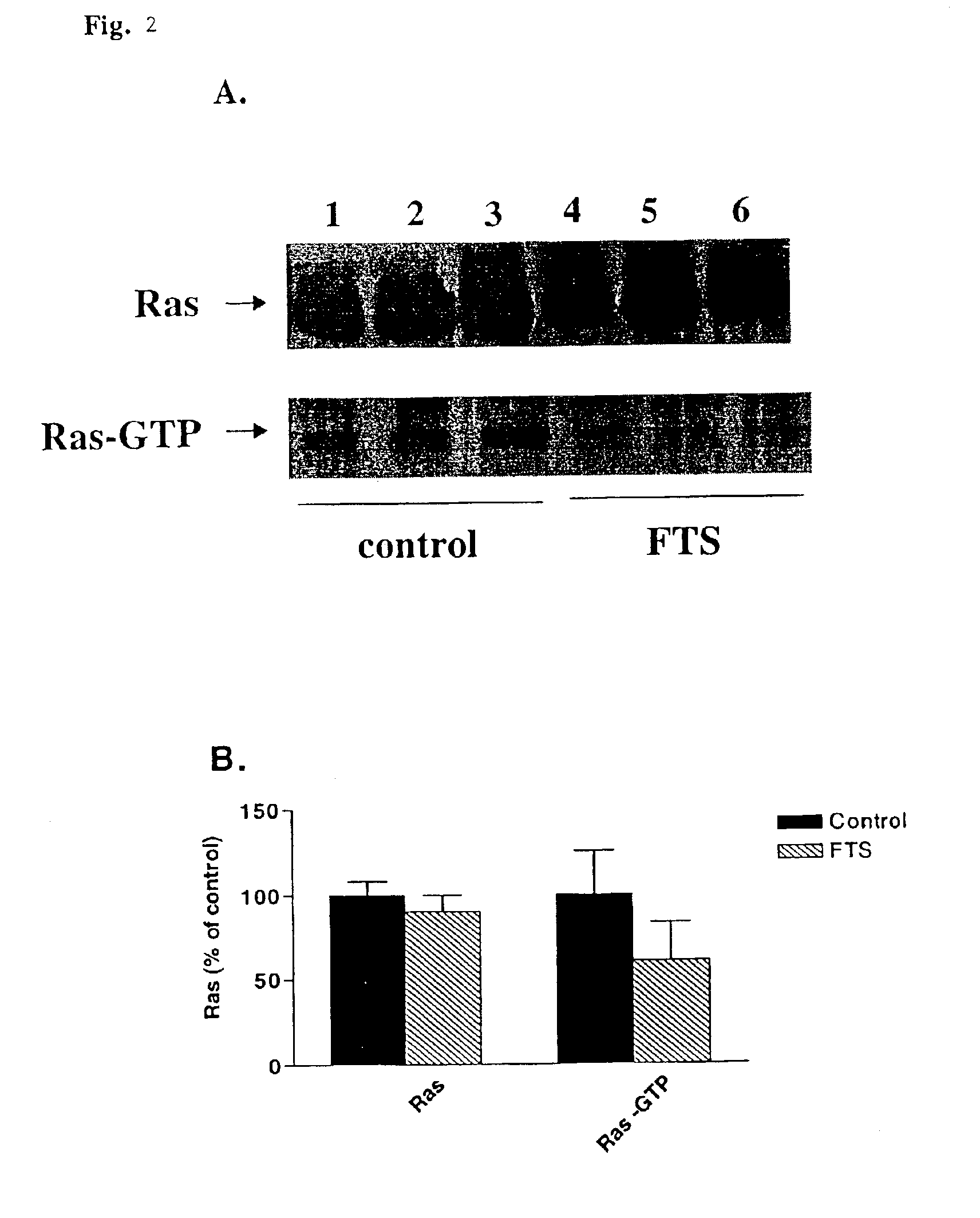
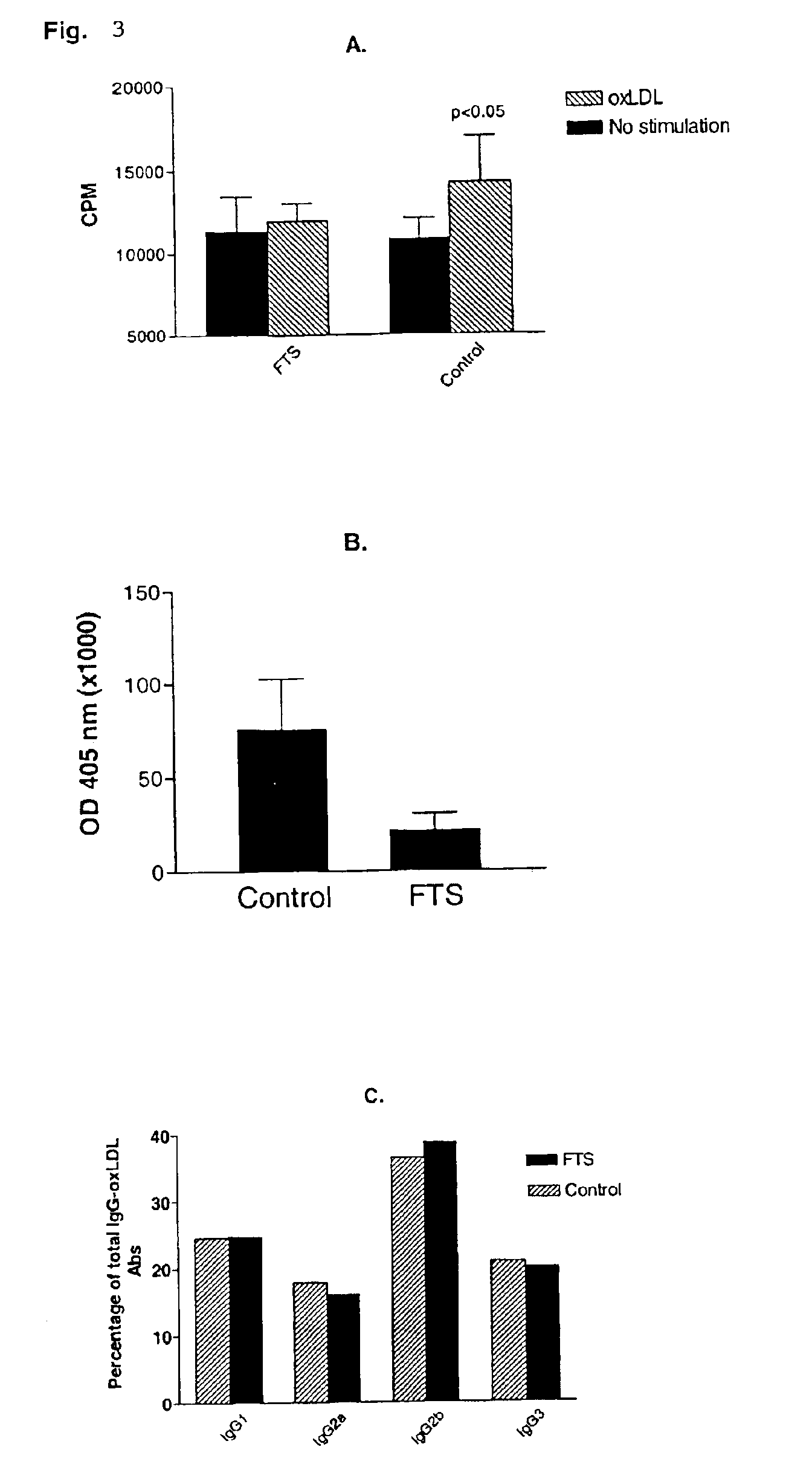
Treatment of post-angioplasty restenosis and atherosclerosis with Ras antagonists
InactiveUS6946485B2Affect growth of cancerReduction in cancer cell growthSalicyclic acid active ingredientsBiocidePercent Diameter StenosisPercutaneous angioplasty
Disclosed are methods for inhibiting Ras activity such as cell proliferation associated with vascular injury such as post-angioplasty restenosis and atherosclerosis. Preferred Ras antagonists are S-trans-trans farnesylthiosalicylic acid (FTS) and structurally related compounds (or analogs) thereof.
Owner:RAMOT AT TEL AVIV UNIV LTD
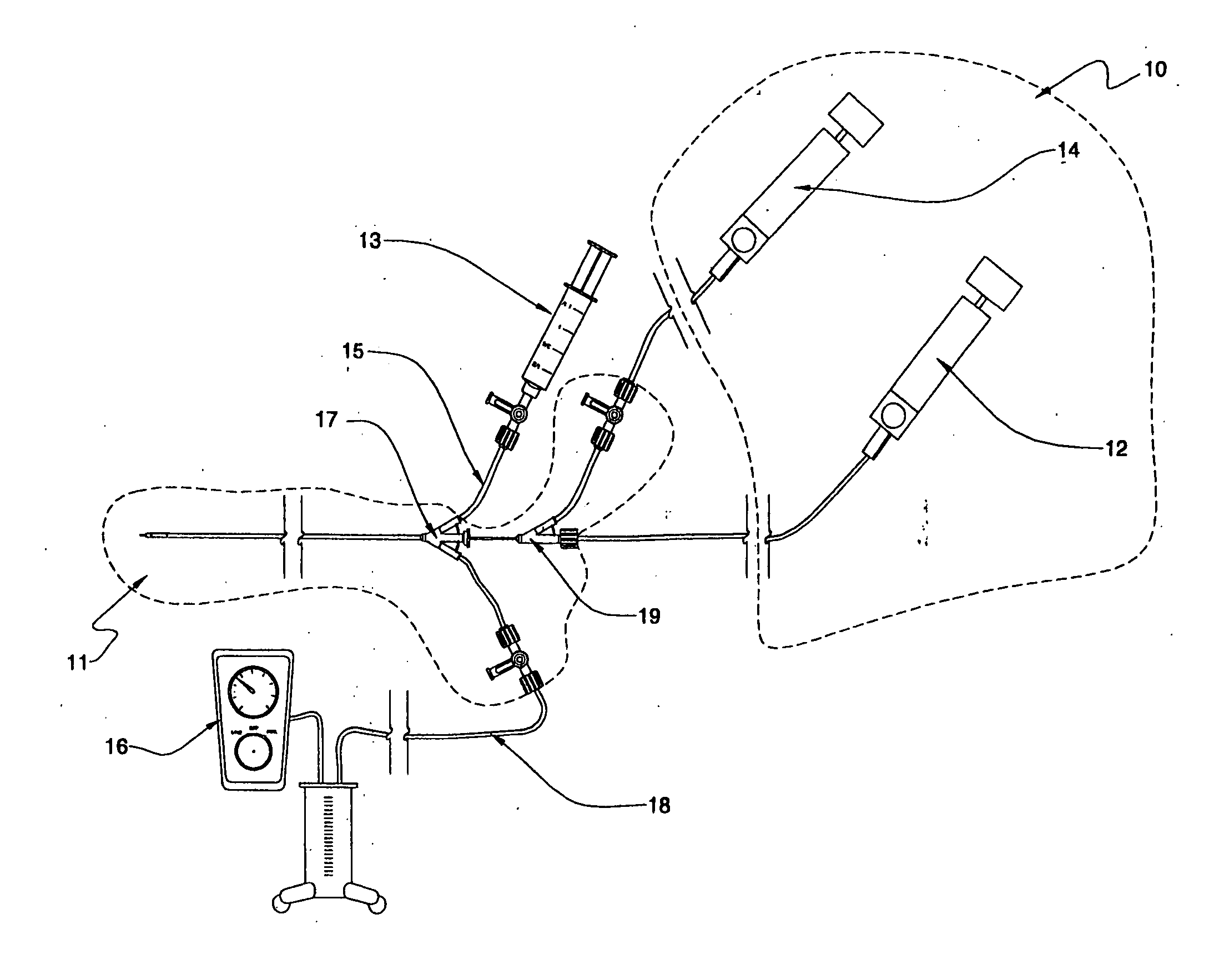
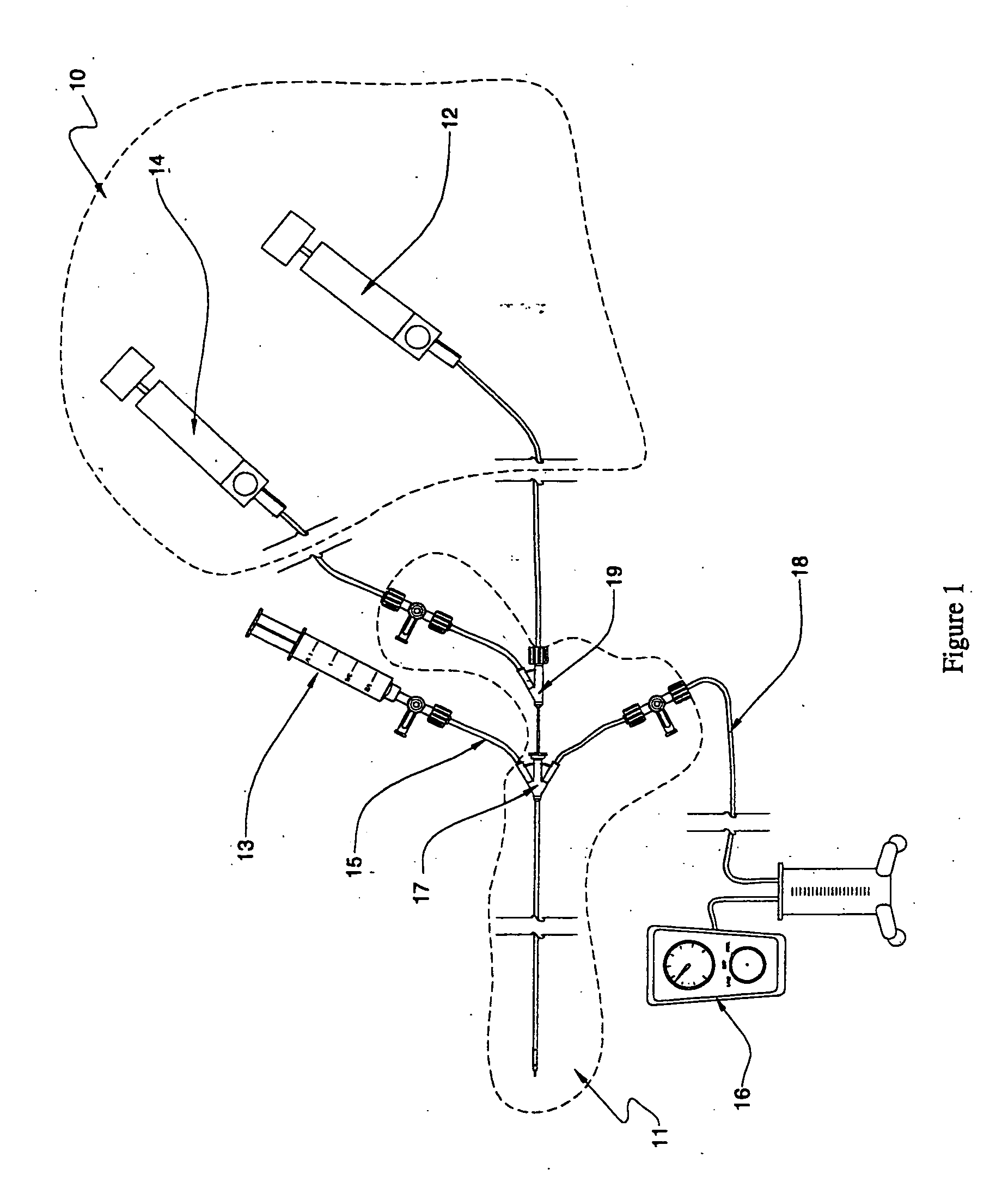
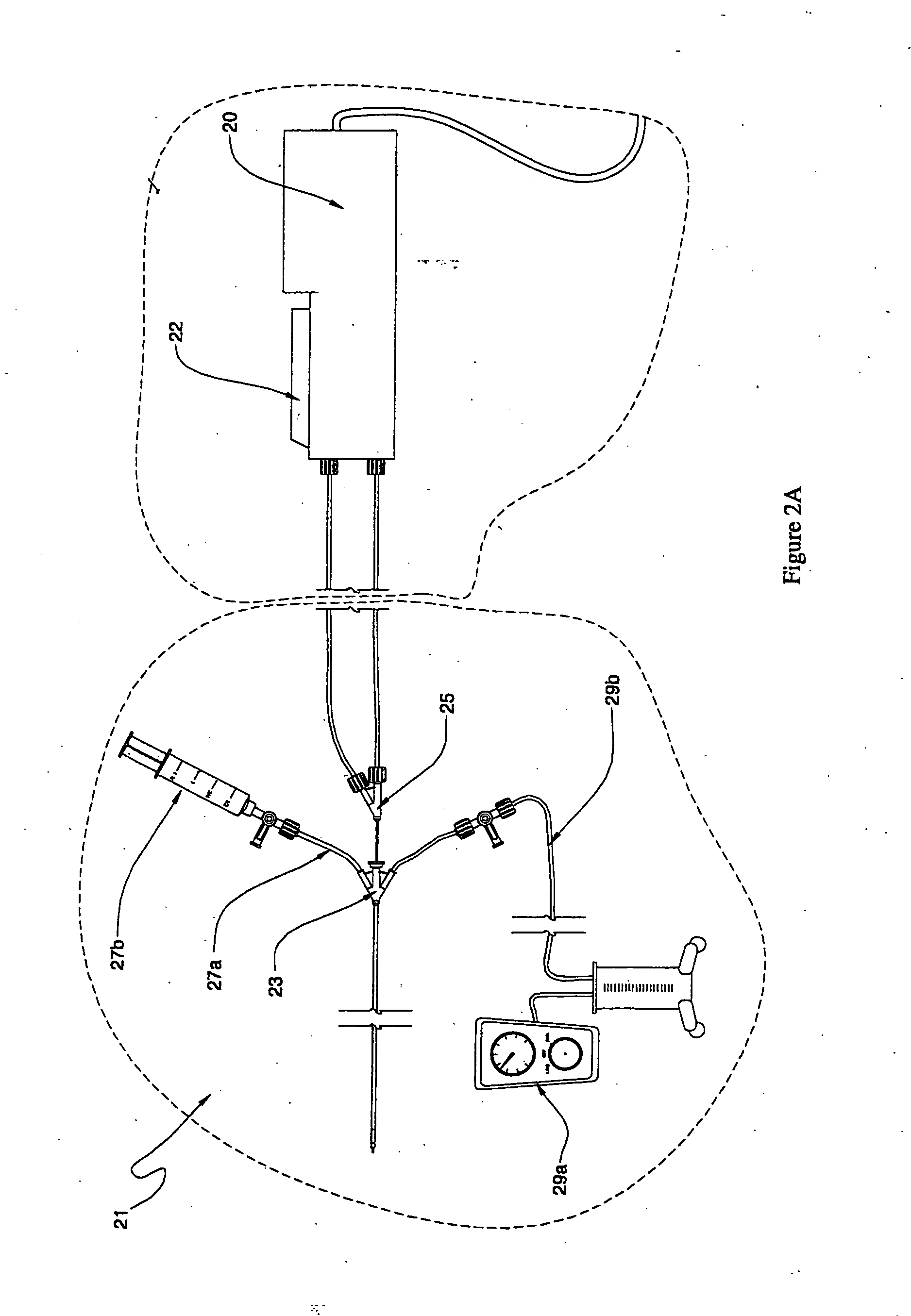
Fluid delivery systems for delivering fluids to multi-lumen catheters
Fluid delivery systems capable of introducing first and second fluids into first and second lumens of a multi-lumen catheter are provided. The first and second fluids are generally a dissolution fluid and a dissolution fluid attenuating fluid. Also provided are fluid delivery devices and kits that include the subject systems. The subject fluid delivery systems find use in a variety of different applications, and are particularly suited for use in the chemical ablation of internal vascular lesions.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
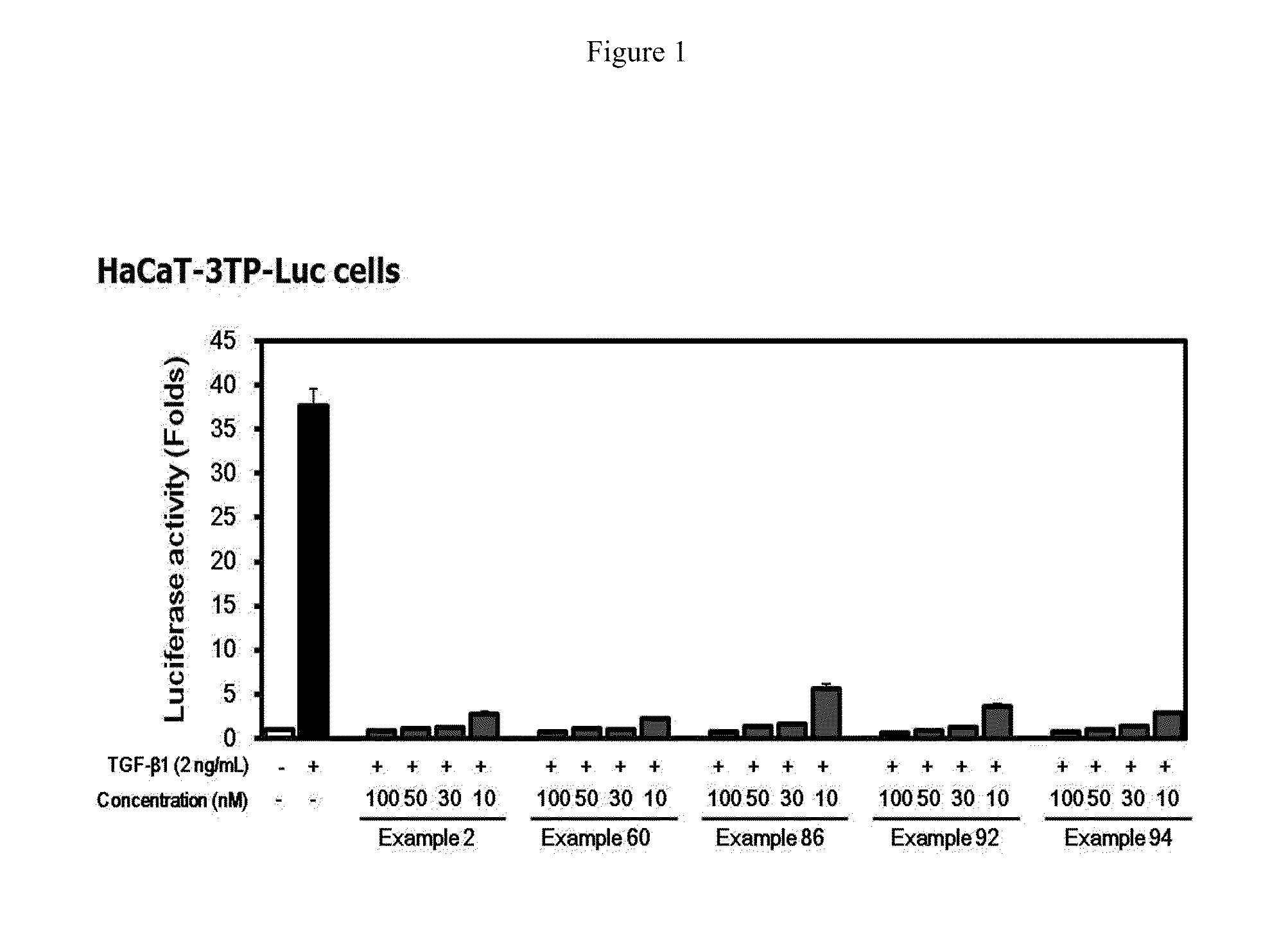
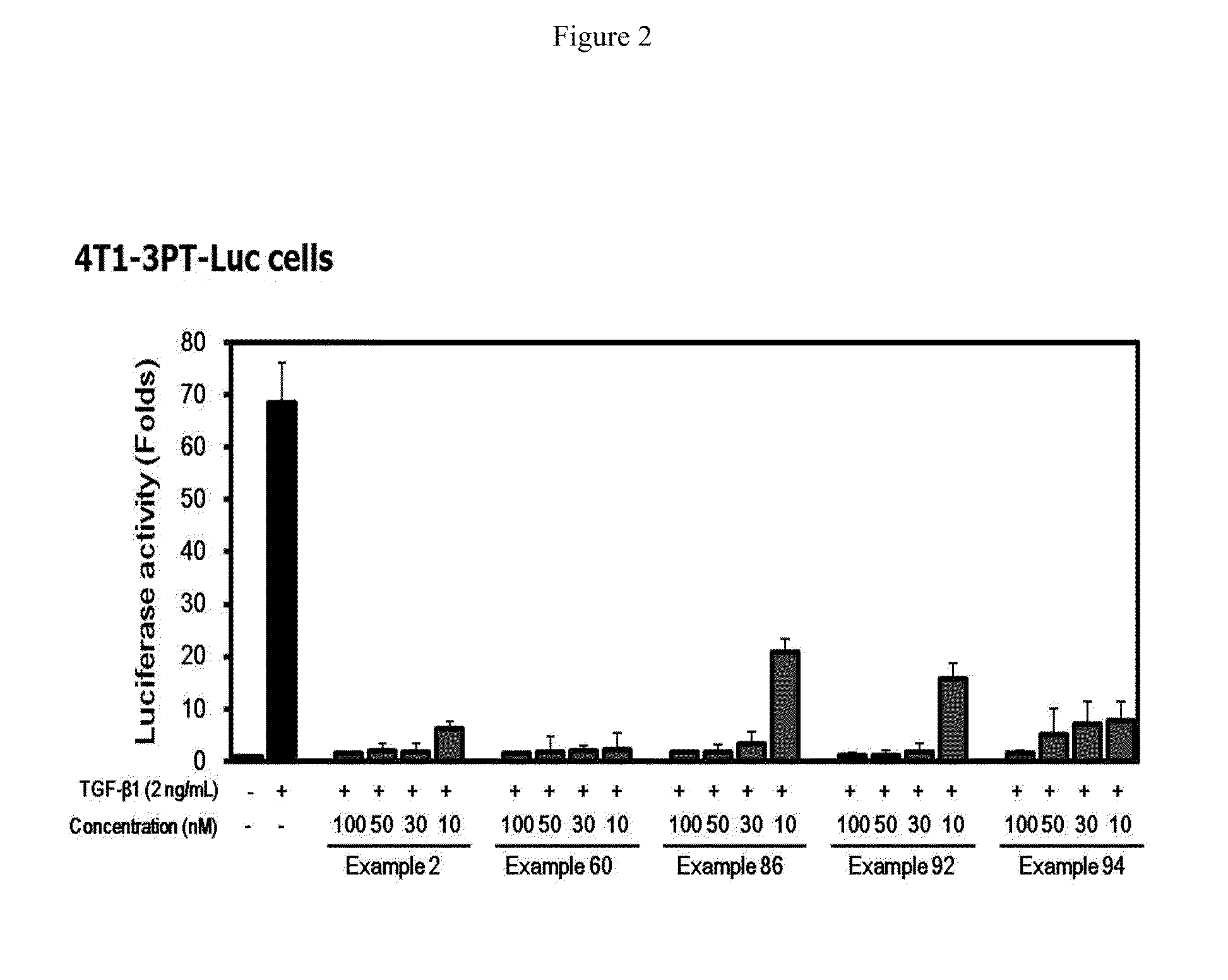
Methods of Treating Fibrosis, Cancer and Vascular Injuries
This invention relates to use of inhibitors of the transforming growth factor-β (TGF-β) type I receptor (ALK5) and / or the activin type I receptor (ALK4) in treating, preventing, or reducing fibrosis, cancer, and vascular injuries.
Owner:EWHA UNIV IND COLLABORATION FOUND
Device and method for inducing vascular injury and/or blockage in an animal model
Ultrashort laser pulses are used to induce photodisruptive breakdown in vasculature in an animal to controllably produce hemorrhage, thrombosis or breach of the blood-brain barrier in individual, specifically-targeted blood vessels. Damage is limited to the targeted vessels such that neighboring vessels exhibit no signs of vascular damage, including vessels directly above and directly below the targeted vessel. Ultrashort laser pulses of lower energy are also used to observe and quantify the baseline and altered states of blood flow. Observation and measurement may be performed by TPLSM, OCT or other known techniques, providing a real-time, in vivo model for the dynamics and effects of vascular injury.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
Infarct area perfusion-improving compositions and methods of vascular injury repair
ActiveUS9034316B2Increase perfusionReduce areaBiocidePeptide/protein ingredientsDiseaseBlood vessel injury
The described invention provides pharmaceutical compositions for treating an infarct area injury and methods of treating or repairing the infarct area injury in a revascularized subject in the aftermath of an acute myocardial infarction resulting from a natural disease process by administering to the subject parenterally through a catheter a sterile pharmaceutical composition containing a therapeutically effective amount of a nonexpanded sterile isolated chemotactic hematopoietic stem cell product as a first therapeutic agent and optionally a therapeutically effective amount of at least one compatible second therapeutic agent. The infarct area-improving amount of the sterile isolated chemotactic hematopoietic stem cell product comprises an enriched population of isolated autologous CD34+ cells containing a subpopulation of potent cells expressing CXCR-4 and having CXCR-4-mediated chemotactic activity such that the enriched population of isolated autologous CD34+ hematopoietic stem cells provides at least 0.5×106 potent CD34+ cells expressing CXCR-4 and having CXCR-4 mediated chemotactic activity.
Owner:CALADRIUS BIOSCI
Method and devices for flow occlusion during device exchanges
A method for facilitating treatment of catheter induced vascular injuries may first involve introducing a guide wire into a vascular sheath residing in a blood vessel, where the guide wire has a distal end and an inflatable balloon at least 15 cm proximal of the distal end. The method may further involve proximally retracting the vascular sheath while leaving the wire in place and observing indicia of the presence or absence of a vascular injury caused to the blood vessel by the vascular sheath or a procedural catheter previously advanced through the vascular sheath. If indicia of a vascular injury are observed, the method may involve positioning the inflatable balloon at or near the vascular injury and inflating the balloon to reduce blood flow past the vascular injury, while leaving the guide wire in place to provide subsequent access to the injury.
Owner:ACCESS CLOSURE INC
Laser treatment of cutaneous vascular lesions
InactiveUS20060069166A1Reduce laser doseFluences for the permanent destruction of blood vessels in the skin are significantly lowerBiocideHydroxy compound active ingredientsDiseaseBlood flow
Owner:VARGAS GRACIE +4
Compositions and methods of vascular injury repair
The present invention relates to pharmaceutical compositions comprising a chemotactic hematopoietic stem cell product comprising an enriched population of CD34+ cells containing a subpopulation of CD34+ / CXCR-4+ cells having CXCR-4-mediated chemotactic activity, methods of preparing these compositions and use of these compositions to treat or repair vascular injury, including infarcted myocardium.
Owner:CALADRIUS BIOSCI
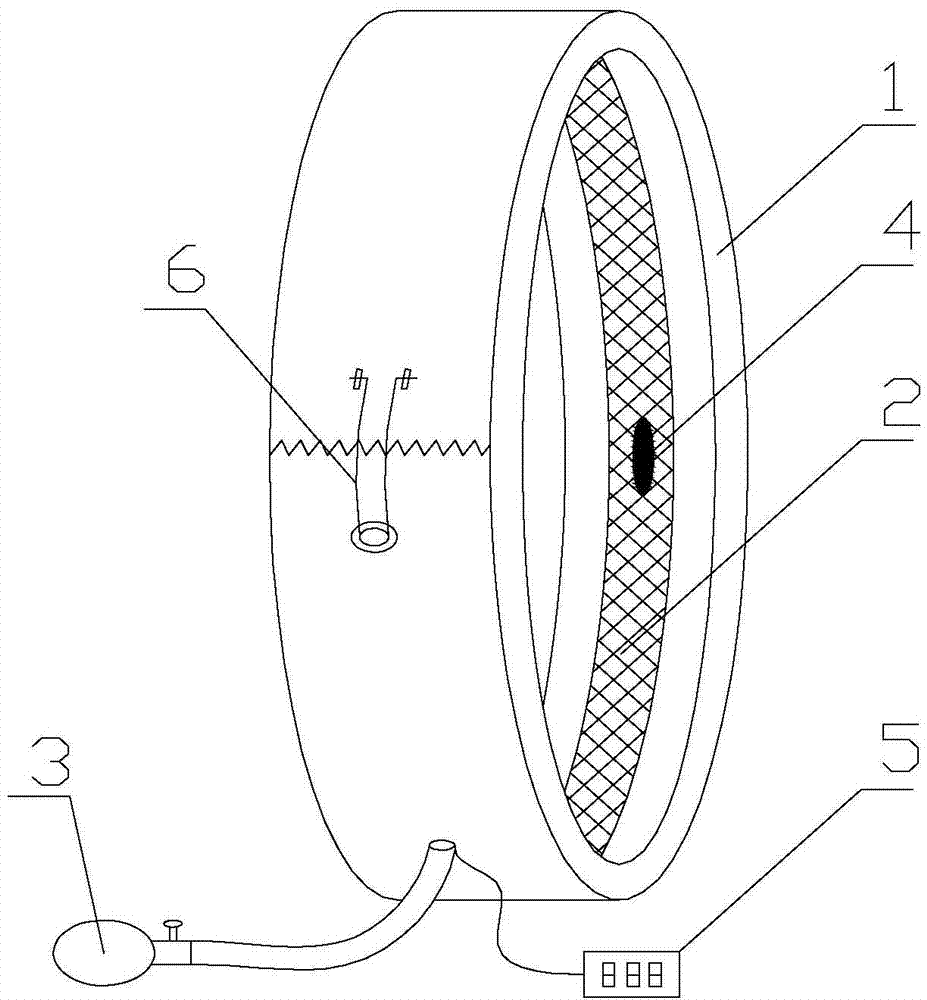
Blood volume controllable vessel occlusion device
The invention discloses a blood volume controllable vessel occlusion device. The blood volume controllable vessel occlusion device comprises a fracture outer ring and an air charging device, wherein the fracture outer ring can be opened and closed, a fracture air bag is fixedly arranged on the inner side of the fracture outer ring along the peripheral direction, the fracture positions of the fracture outer ring and the fracture air bag coincide to achieve simultaneous opening and closing, and the air charging device is communicated with the air bag. An operator can pressurize a blood vessel through the air bag according to practical need, and the pressure borne by the vessel at the time when the vessel is occluded, namely, the measured pressure value, can be read on a pressure display device, so as to judge the degree, by which the blood flow is occluded. By adopting the vessel occlusion device, not only can the blood flow occlusion to any degree be achieved according to practical need in an operation so as to more accurately and more intuitively measure the blood flow occlusion conditions for the blood vessels at different thicknesses and types, but also the blood flow occlusion manner is less in injury on the blood vessel to achieve the reversible operation on increasing and reducing the blood flow amount so as to facilitate the blood flow detection relevant assay or treatment control.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Absorbable intravascular stent and preparation method thereof
PendingCN107670121AImprove corrosion resistanceGood biocompatibilityStentsBile ductsBlood vessel injuryIntravascular stent
The invention belongs to the technical field of medical apparatus and instruments, and particularly relates to an absorbable intravascular stent and a preparation method thereof. The absorbable intravascular stent comprises a net-shaped degradable metal stent body and a degradable polymer coating which is arranged on the surface of netting wires of the net-shaped degradable metal stent body; and the component of the degradable polymer coating is polylactic acid-hydroxyacetic acid copolymer. The degradation time of the absorbable intravascular stent can be correspondingly regulated and controlled by adjustment of the components of a stent structure and the component of the degradable polymer coating, the components can be degraded and discharged out of the body, the shortest period of degradation and discharging is within 1-2 months, the longest period of degradation and discharging can be 12 months, the degradation time can be adjusted according to specific conditions, the circumstances that foreign body sensation is caused by existing of the stent after the task of the stent is finished, and vascular injury is caused by the stent are avoided, and meanwhile, a series of problems ofcoating, displacement and the like which are caused by stimulation on an immune system due to the unnecessary stent are further avoided.
Owner:北京赛铂医药科技有限公司
Biological markers and response to treatment for pain, inflammation, neuronal or vascular injury and methods of use
The invention provides methods and kits for treatment of pain, inflammation, neuronal or vascular injury and the use of biomarkers for the assessment of the biological activity or disease state. In one embodiment, the kit comprises a biomembrane sealing agent, such as PEG, a bioactive agent, such as a magnesium compound, and an assay for measuring a biomarker. The biomarker is measured pre-, mid- and post-treatment, and results of the measurements are compared to evaluate the treatment, treatment regimen and outcomes.
Owner:WARSAW ORTHOPEDIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com