Compositions and methods of vascular injury repair
a technology of hematopoietic stem cells and compositions, applied in the direction of drug compositions, extracellular fluid disorders, catheters, etc., can solve the problems of permanent cardiac dysfunction, increased lifetime risk of experiencing adverse cardiac events, and further demise of cardiac function, so as to achieve the sterility of the chemotactic hematopoietic cell produ
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Selection of Eligible Subjects
[0164]Subjects / patients presenting with symptoms and clinical findings suggestive of a myocardial infarction will receive emergency diagnostic and clinical management according to institutional guidelines. If a transmural (meaning through the wall) myocardial infarction is confirmed, the time of first symptoms and the time of successful stent placement will be recorded. Revascularized subjects will receive appropriate medical management to reduce ventricular wall stresses according to institutional guidelines. The term “revascularized” as used in this embodiment, refers to the successful placement of a stent.
[0165]All types of stents, including drug-eluting stents (e.g., paclitaxel or sirolimus) are acceptable for use in the revascularization of the infarct related artery (“IRA”). Previous studies employing balloon catheters to infuse cell products have reported no limits for reference vessel diameter for the placement of the stent. Since this study is ...
example 2
[0177]Sterile Preparation and Draping
[0178]The subject will be brought into the Cardiac Catheterization Laboratory after the investigator has obtained an informed consent. The subject will receive a sterile preparation and draping in the Cardiac Catheterization Laboratory.
[0179]Cardiac Catheterization
[0180]Vascular access will be obtained by standard technique using right or left groin. A sheath will be placed in the femoral artery or the right or left brachial artery. Coronary arteriographic examination will be performed by obtaining standard views of both right and left coronary arteries. Multiple views will be obtained to identify the previously stented infarct related artery. All subjects will receive standard medications during the catheterization procedure in accordance with routine practice.
example 3
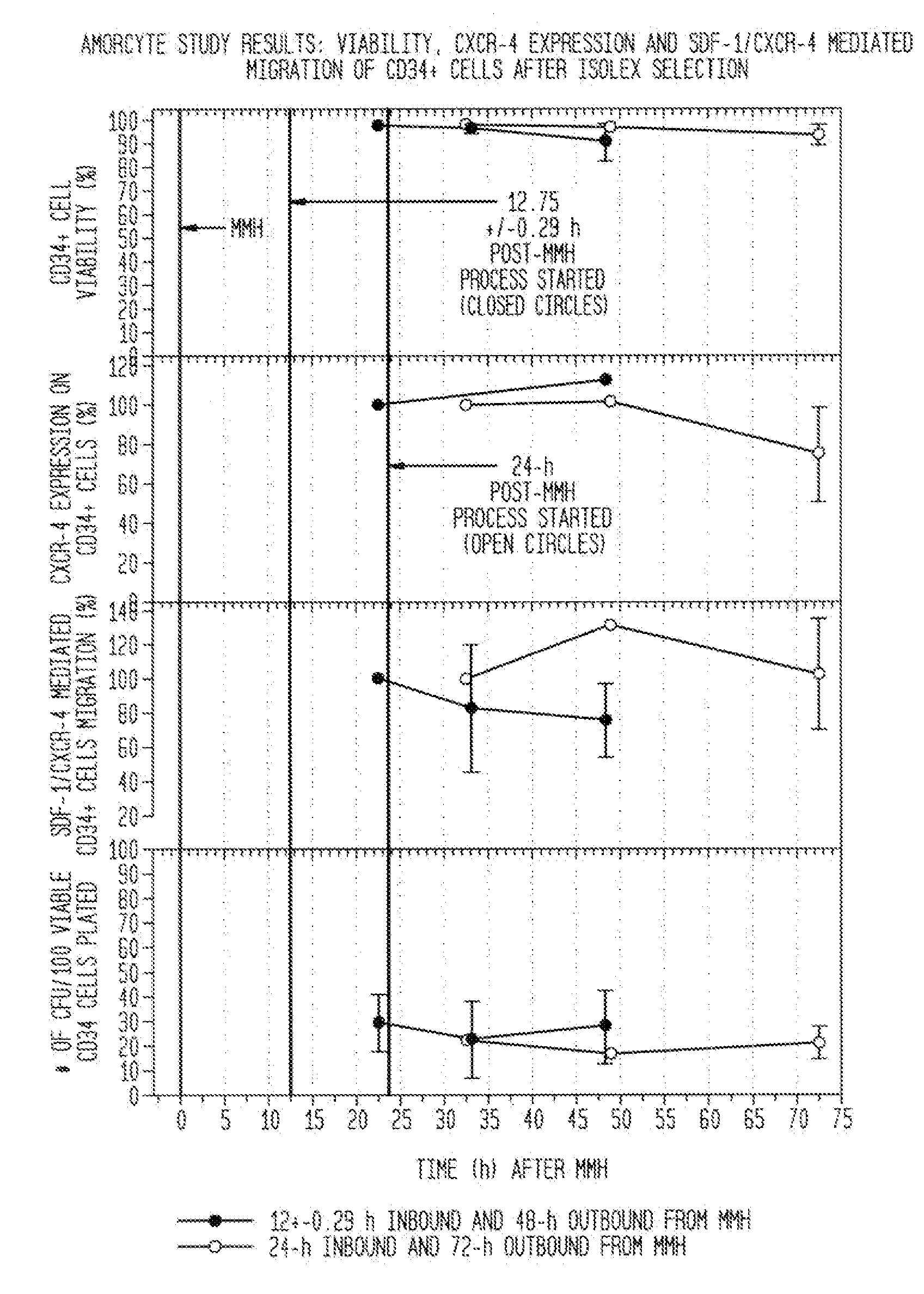
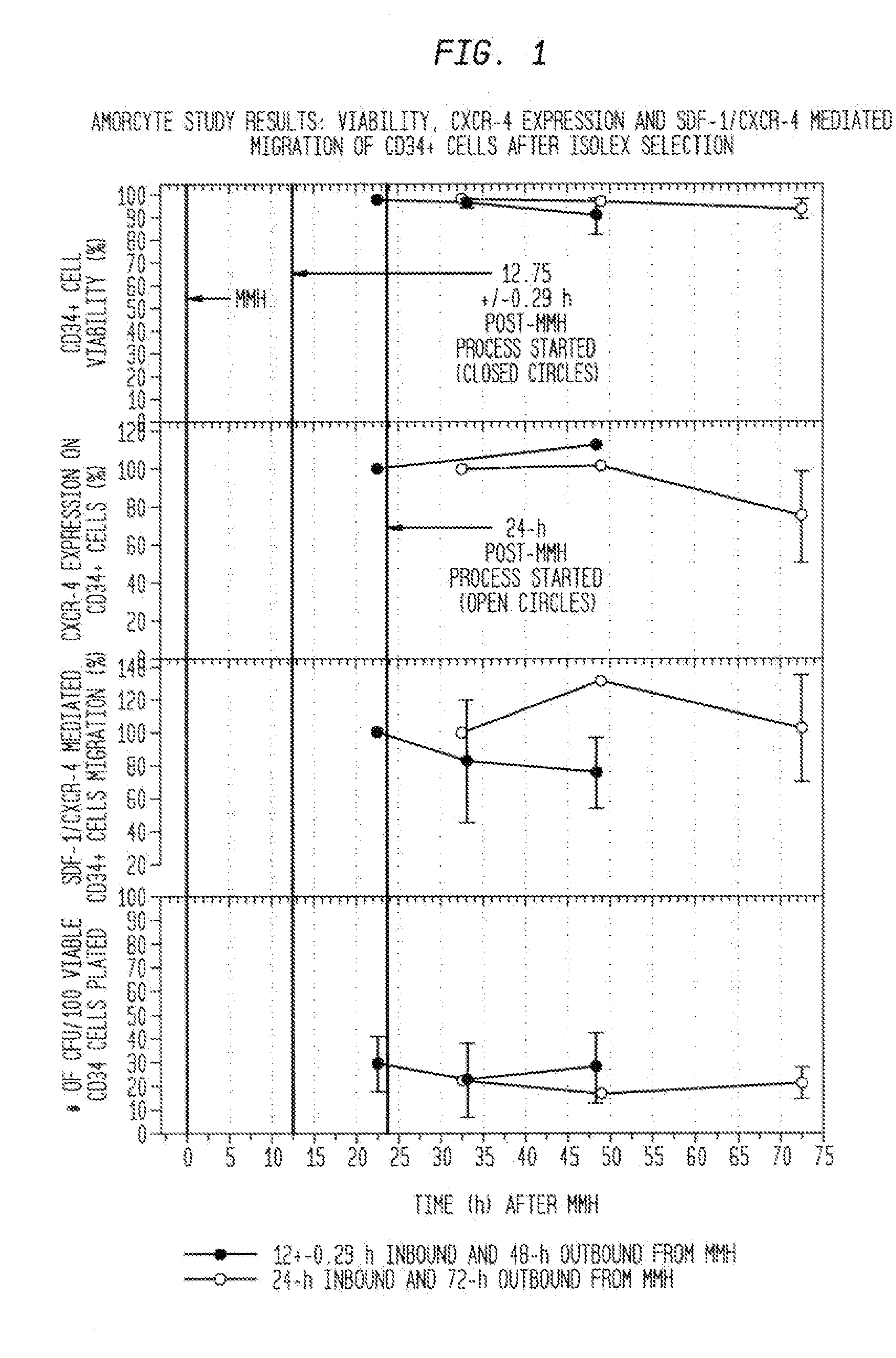
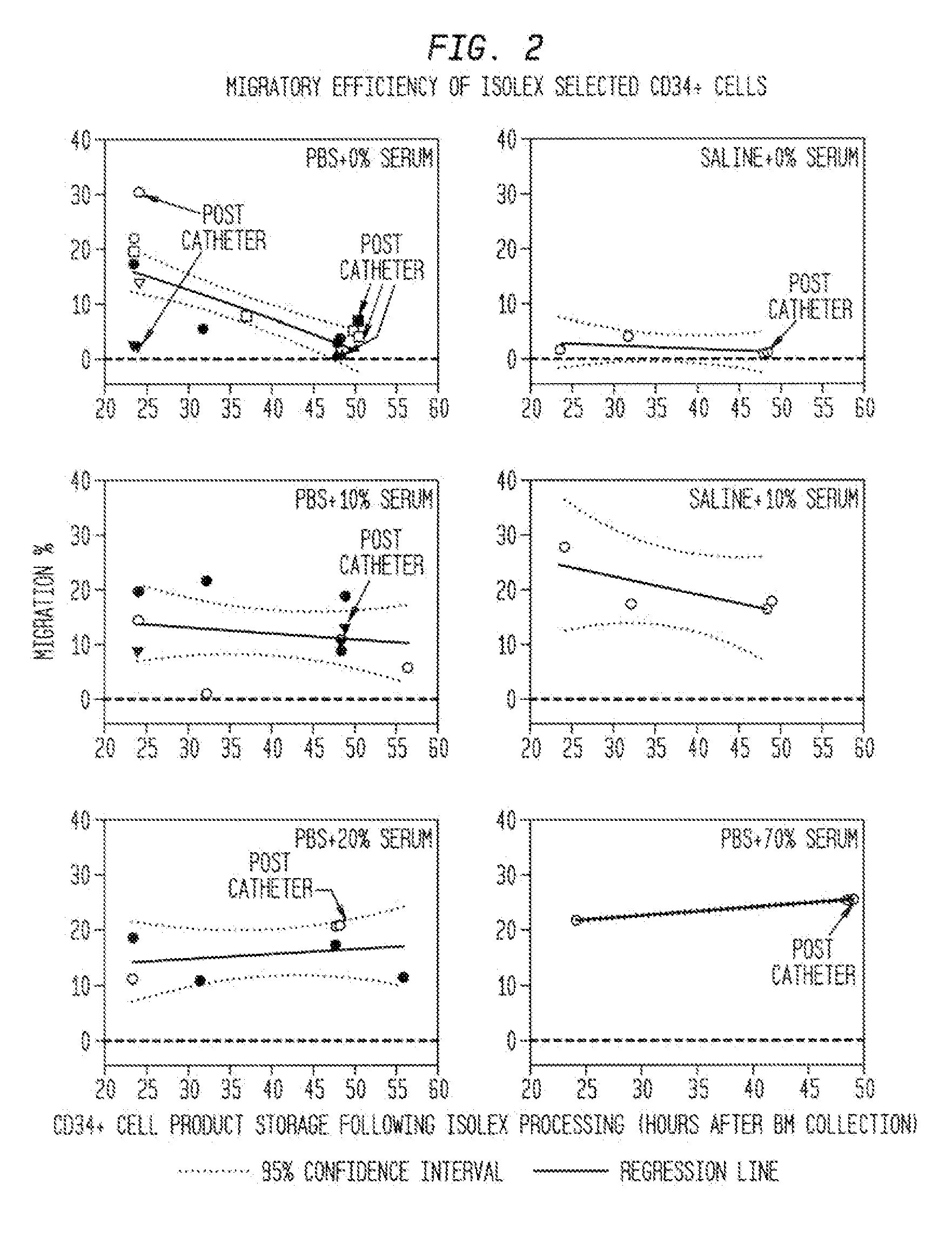
Acquisition Process For Acquiring Chemotactic Hematopoietic Stem Cell Product That Can Then Be Enriched For CD34+ Cells
[0181]While it is contemplated that any acquisition process appropriate for acquiring the chemotactic hematopoietic stem cell product comprising potent CD34+ cells is within the scope of the present invention, the following example illustrates one such process referred to herein as a mini-bone marrow harvest technique.
[0182]Preparation of Harvesting Syringes
[0183]According to one embodiment, prior to the bone marrow harvest, forty 10 cc syringes optionally loaded with about 2-ml of a preservative free heparinized saline solution (about 100 units / ml to about 125 units / ml, APP Cat. No. 42592B or equivalent) will be prepared under sterile conditions. According to one embodiment, heparin will be injected via a sterile port into each of two 100-ml bags of sterile 0.9% normal saline solution (“Normal Saline”, Hospira Cat. No. 7983-09 or equivalent) following removal of 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com