Tanshinone II A sodium sulfonate and method of determining tanshinone I sodium sulfonate in its preparation
A method of determination, the technology of sodium sulfonate, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of measurement content deviation, poor reproducibility and quantitative accuracy, and low sensitivity of impurity detection, and achieve the goal of measuring easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
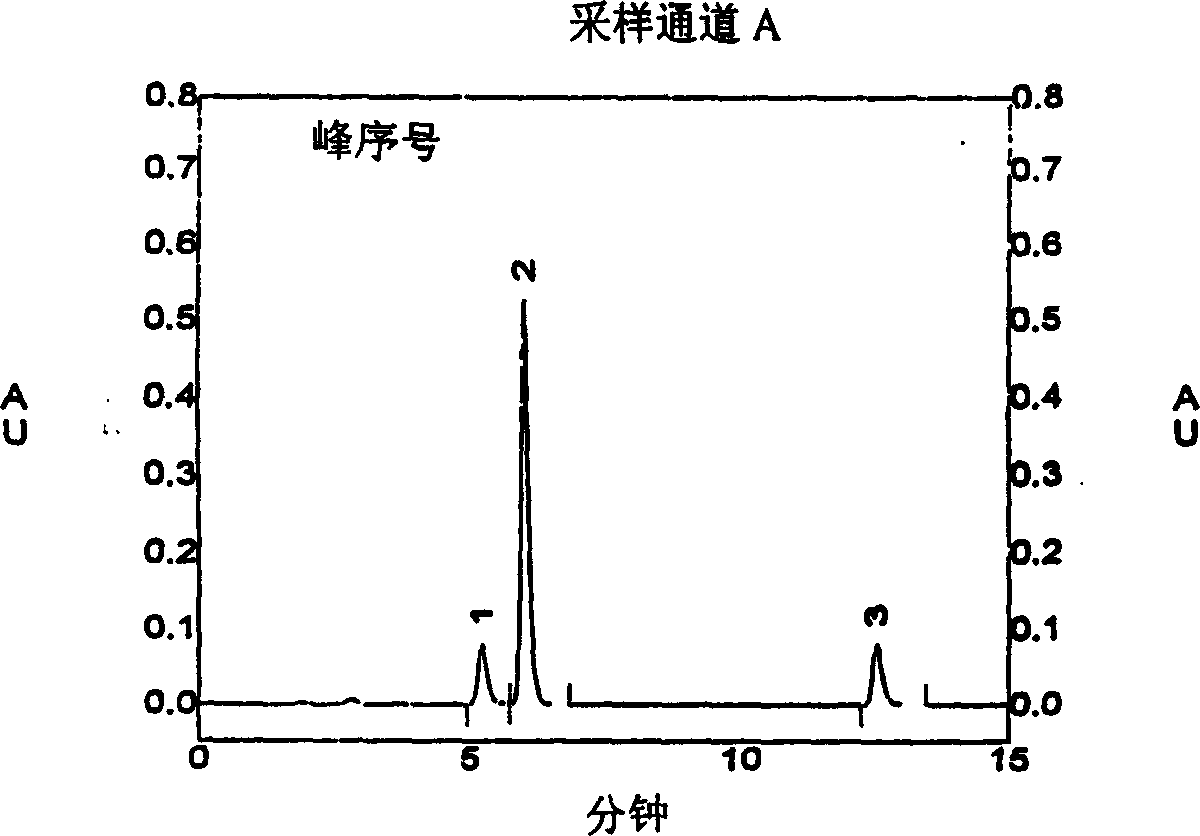
[0020] Example 1 Chromatographic conditions Chromatographic column: diamonsil ODS (250×4.6mm, 5 μm); mobile phase: acetonitrile-phosphate buffer (take 2.0 g of potassium dihydrogen phosphate, add 1000 ml of water, adjust the pH value to 3.0 with phosphoric acid) (two Component volume ratio 8:2), flow rate: 1ml / min; detection wavelength: 270nm; injection volume 20μl. Under the chromatographic conditions, the retention times of sodium tanshinone I sulfonate, sodium tanshinone IIA sulfonate, and tanshinone IIA were 5.2 minutes, 6.0 minutes, and 12.5 minutes, respectively, and the substances were well separated. The peak area of sodium tanshinone I sulfonate was plotted against the concentration, and the regression equation and correlation coefficient were calculated by the least square method. The correlation coefficient r=0.9994, indicating that the peak area had a good linear relationship with the concentration. (See Attachment 1)
Embodiment 2
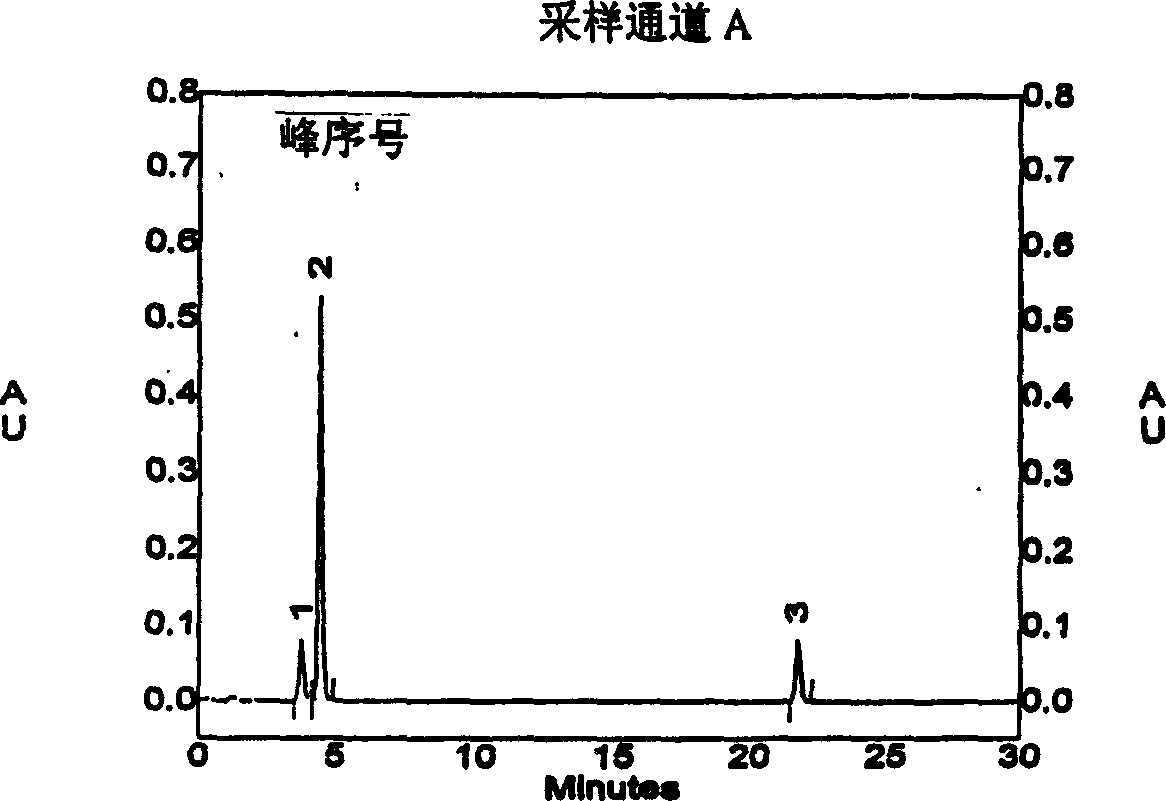
[0021] Example 2 Chromatographic conditions Chromatographic column: symmetryshield RP 18 (3.9×150mm, 5μm); mobile phase: methanol-phosphate buffer (take 3.45g of ammonium dihydrogen phosphate, add 1000ml of water, adjust the pH value to 3.3 with phosphoric acid) (volume ratio of the two components is 65:35), flow rate: 1ml / min; detection wavelength: 270nm; injection volume 20μl. Under these chromatographic conditions, the retention times of sodium tanshinone I sulfonate, sodium tanshinone IIA sulfonate and tanshinone IIA were 3.7 minutes, 4.4 minutes and 21.8 minutes, respectively, and the substances were well separated. The peak area of tanshinone I sulfonate sodium was plotted against the concentration, and the regression equation and correlation coefficient were calculated by the least square method. The correlation coefficient r=0.9992, indicating that the peak area had a good linear relationship with the concentration. (See Attachment 2)
Embodiment 3
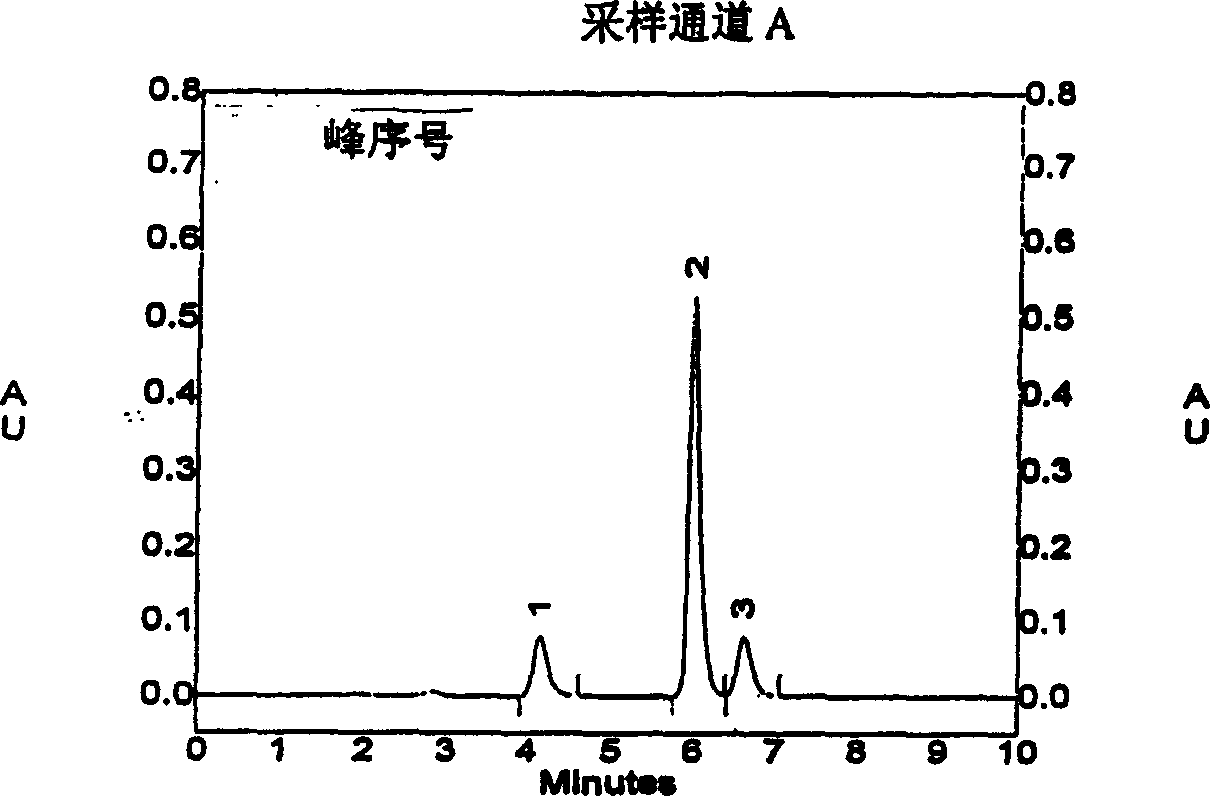
[0022] Example 3 Chromatographic conditions Chromatographic column: inertsil CN-3 (4.6×150mm, 5μm); mobile phase: methanol-phosphate buffer (take 3.45g of ammonium dihydrogen phosphate, add 1000ml of water, adjust the pH value to 3.3 with phosphoric acid) ( The volume ratio of the two components is 65:35), the flow rate: 1ml / min; the detection wavelength: 270nm; the injection volume is 20μl. Under these chromatographic conditions, the retention times of tanshinone IIA, sodium tanshinone IIA sulfonate and sodium tanshinone I sulfonate were 4.1 minutes, 6.0 minutes and 6.6 minutes, respectively, and the substances were well separated. The peak area of sodium tanshinone I sulfonate was plotted against the concentration, and the regression equation and correlation coefficient were calculated by the least square method. The correlation coefficient r=0.9987, indicating that the peak area had a good linear relationship with the concentration. (See Attachment 3)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com