Synthesis and application of sulfonamide compounds
A technology of sulfonyl carbonamide and sulfonamide carbonamide, which is applied in the field of medicine, can solve the problems of high irritation of products, pain of patients, and low pH value of injections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
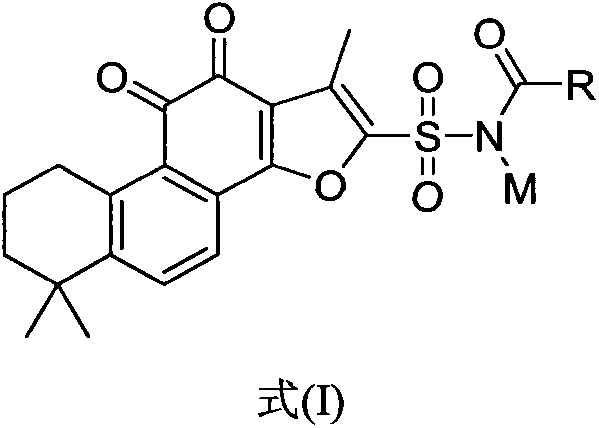
[0039] [Example 1]: N-(1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11-hexahydrophenanthrene[1,2-b] Preparation of furan-2-ylsulfonyl)acetamide sodium salt
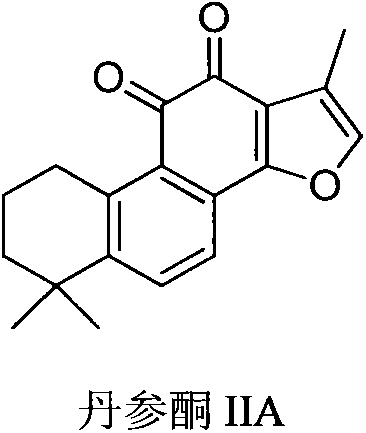
[0040] Step 1: Preparation of Intermediate-1 Tanshinone IIA Sulfonyl Chloride
[0041] Tanshinone IIA (10 g, 33 mmol) was added dropwise into 150 mL of sulfuryl chloride, and refluxed for 1 hour. The temperature of the reaction system was lowered to room temperature, and the unconsumed sulfuryl chloride was distilled off under reduced pressure. The remaining black oily liquid was dried under reduced pressure with an oil pump for 2 hours under the condition of avoiding light, and the obtained black oily liquid was directly used in the next reaction.
[0042] Step 2: Preparation of Tanshinone IIA Sulfonamide
[0043] The product obtained in Step 1 (1 g, 2.55 mmol) was dissolved in 10 mL of THF, and added dropwise to vigorously stirred 0-degree ammonia water (10 mL). After the dropwise addition was complete, stir at room...
Embodiment 2
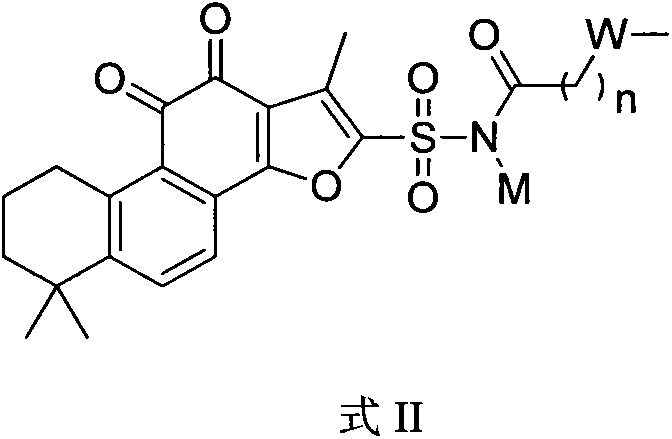
[0048] Example [2]: N-(1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11-hexahydrophenanthrene [ 1,2-b] preparation of furan-2-ylsulfonyl)propionamide sodium salt
Embodiment 1
[0049] According to the preparation method of Example [1], replace acetyl chloride with propionyl chloride to obtain N-(1,6,6-trimethyl-10,11-dioxo-6,7,8,9,10,11 - Hexahydrophenanthrene[1,2-b]furan-2-ylsulfonyl)propionamide sodium salt. 1 H NMR (400MHz, D 2 O) δ7.90-7.86(m, 2H), 3.20(m, 2H), 2.52(s, 3H), 2.01(m, 2H), 1.60(m, 2H), 1.53(m, 2H), 1.29( s, 6H), 1.11(t, 3H), LCMS m / z, 428.1(M-1) +
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com