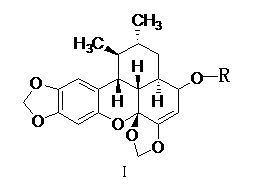
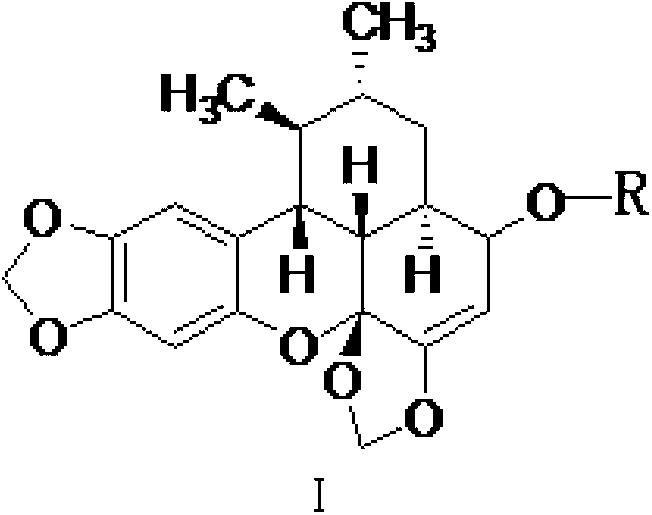
Sauchinone derivative and preparing method and application thereof
A technology of salicylicone and derivatives, applied in the field of medicinal chemistry research, can solve the problems of low adverse reactions and the like, and achieve the effects of low adverse reactions, high purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
[0030] Example 1 Preparation of Tribaurumone Derivatives (1)
[0031] Take 1g (2.8mmol) of tribauronone in a 100mL round bottom flask, add 20mL of tetrahydrofuran to dissolve; put 0.54g (14mmol) of NaBH 4 Slowly dissolve in 20mL methanol solution to which 20mg NaOH has been added, drop the methanol solution dropwise into the tetrahydrofuran solution of tribagudone that has been pre-cooled in an ice-water bath, react in the ice-water bath for 0.5h, remove the ice bath, and naturally The temperature was raised to room temperature, and the progress of the reaction was detected by thin-layer chromatography, and the reaction was complete after about 3 hours. The reaction solution was rotary evaporated to dryness at 40°C, and the solid was dispersed by ultrasonic dispersion with 100 mL of water, filtered, and the obtained solid was dried and then subjected to silica gel column chromatography, eluting with petroleum ether: ethyl acetate = 10:1, to obtain the tribaquidone derivative (...
Embodiment 2
[0038] Preparation of embodiment 2 derivative (2)
[0039] Add 100mg (0.28mmol) of tribagudone derivative (1) prepared according to the method in the example to a 50mL round-bottomed flask, add 3mL of pyridine to dissolve, then add 0.26mL (2.8mmol) of acetic anhydride, and add 1mg 4 dropwise - 0.5mL pyridine solution of dimethylaminopyridine (DMAP) was catalyzed, reacted at room temperature for 24h, and detected the end point by TLC. After the reaction, wash with 50 mL of distilled water, add 30 mL of ethyl acetate to extract twice, combine the organic phase, wash the organic phase with 30 mL of 1mol / L hydrochloric acid to remove pyridine and DMAP, and wash with 30 mL of saturated NaHCO 3 The solution neutralized the excess acid, and then washed with saturated NaCl until the solution was neutral, and the organic phase was washed with anhydrous NaCl 2 SO 4 Dry, filter, and concentrate to dryness under reduced pressure. Add a small amount of ethanol for recrystallization to o...
Embodiment 3
[0044] Example 3 Pharmacodynamic evaluation of mouse liver injury caused by carbon tetrachloride
[0045] A. Take 50 mice and randomly divide them into 6 groups, namely normal group, model group, and tribaquidone 10, 20, 50 mg / kg dosage groups, with 10 mice in each group. After three days of adaptive feeding, except the normal group and the model group were given the same volume of 0.5% sodium carboxymethyl cellulose solution for intragastric administration, the other groups were given different doses of tribaquidone by intragastric administration, once a day, continuously 7 days. One hour after the last administration, mice in other groups were intraperitoneally injected with 0.1% CCl except for the normal group given an equal volume of peanut oil. 4 Peanut oil 10mL / kg, then fasting without food and water, after an interval of 16 hours, blood was taken from the orbital vein, the blood sample was placed in a water bath at 37°C for 30 minutes, then centrifuged at 3500r / min for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com