Compound angelica medicament injection preparation containing polyethylene glycol 12-hydroxystearate and preparation method thereof
A polyethylene glycol lauryl hydroxystearate and injection preparation technology, which is applied in the field of medicine, can solve the problems of white lumps, solution turbidity, small white spots, etc., and achieve the effect of improving clarity, facilitating clinical medication and popularization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Angelica 100g
[0025] Chuanxiong 100g
[0026] Safflower 100g
[0027] Solutol HS- 2.0g
[0028]Add water to decoct the above three flavors, Angelica and Chuanxiong twice, each time for 2 hours, combine the decoction, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.27-1.30 (80°C), let it cool, add ethanol to make the ethanol content 65%, stand overnight, filter, the filtrate is decompressed to recover ethanol and concentrate to a relative density of 1.30 (80°C), add 3 times the amount of water, stir well, refrigerate for 48 hours, filter, and the filtrate is concentrated to a relative density under reduced pressure About 1.15 (80°C), the medicinal liquid is for later use; add water to decoct the safflower twice, 1 hour each time, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.25-1.30 (80°C), let it cool, add Ethanol so that the ethanol content is 65%, let stand overnight, filter, the fi...
Embodiment 2
[0030] Angelica 50-150g
[0031] Chuanxiong 50-150g
[0032] Safflower 50-150g
[0033] Solutol HS- 1-10g
[0034] Add water to decoct the above three flavors, Angelica and Chuanxiong twice, each time for 2 hours, combine the decoction, filter, and concentrate the filtrate under reduced pressure to a relative density of 1.27-1.30 (80°C), let it cool, add ethanol to make the ethanol content 65%, stand overnight, filter, the filtrate is decompressed to recover ethanol and concentrate to a relative density of 1.30 (80°C), add 3 times the amount of water, stir well, refrigerate for 48 hours, filter, and the filtrate is concentrated to a relative density under reduced pressure About 1.15 (80°C), the medicinal liquid is for later use; add water to decoct the safflower twice, 1 hour each time, combine the decoction, filter, and concentrate the filtrate to a relative density of 1.25-1.30 (80°C), let it cool, add Ethanol so that the ethanol content is 65%, let stand overnight, fil...
Embodiment 3
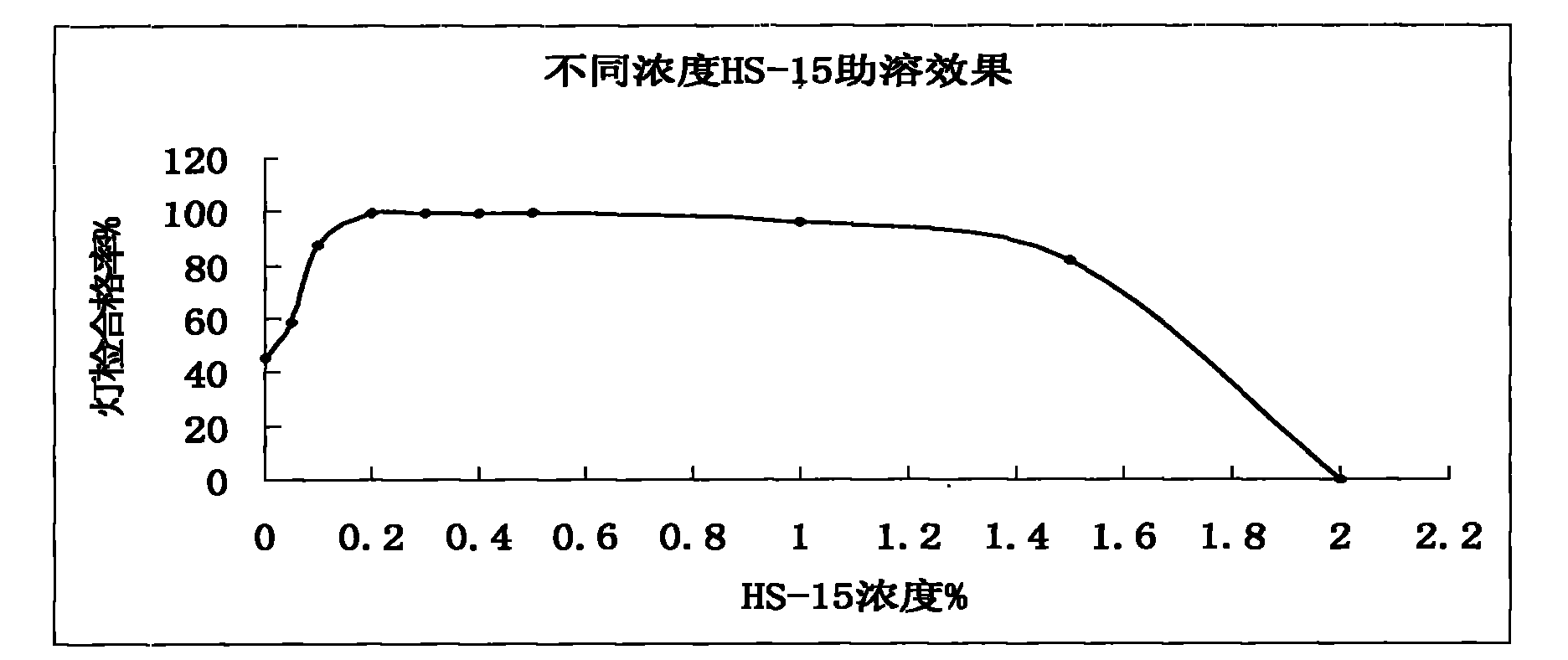
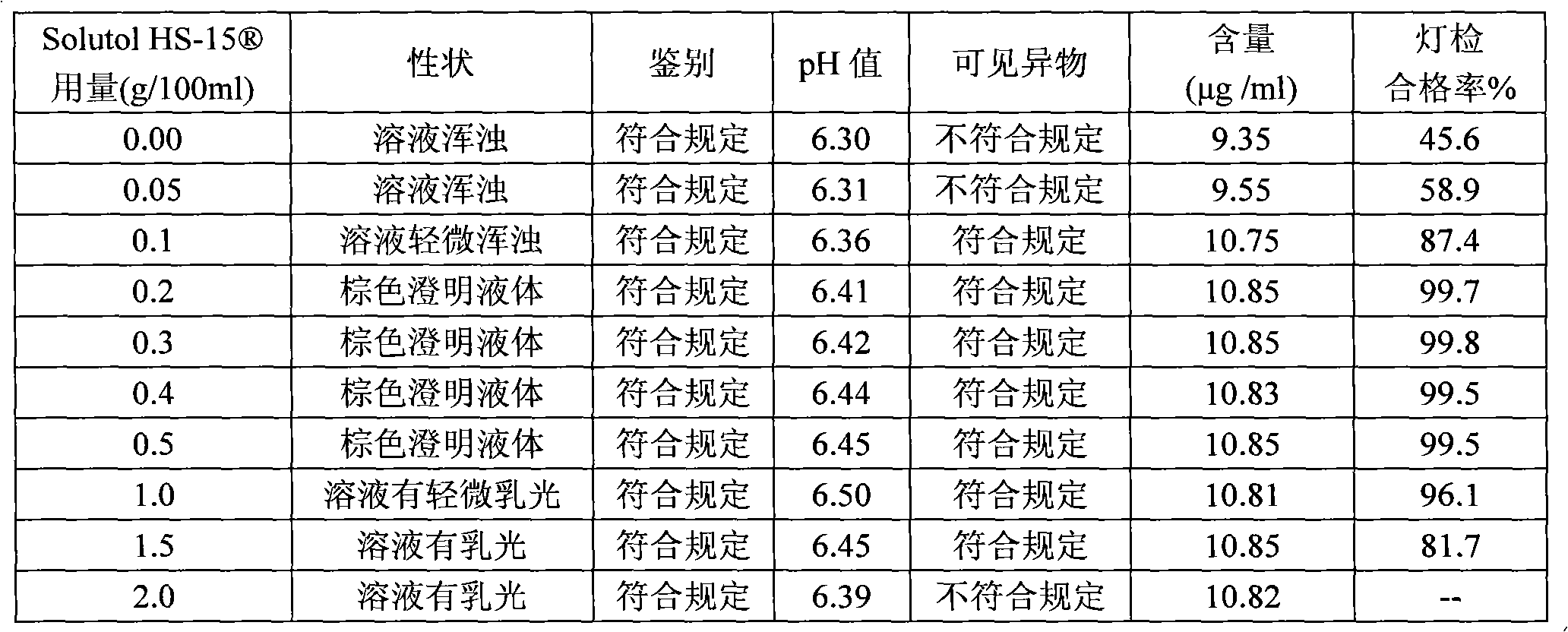
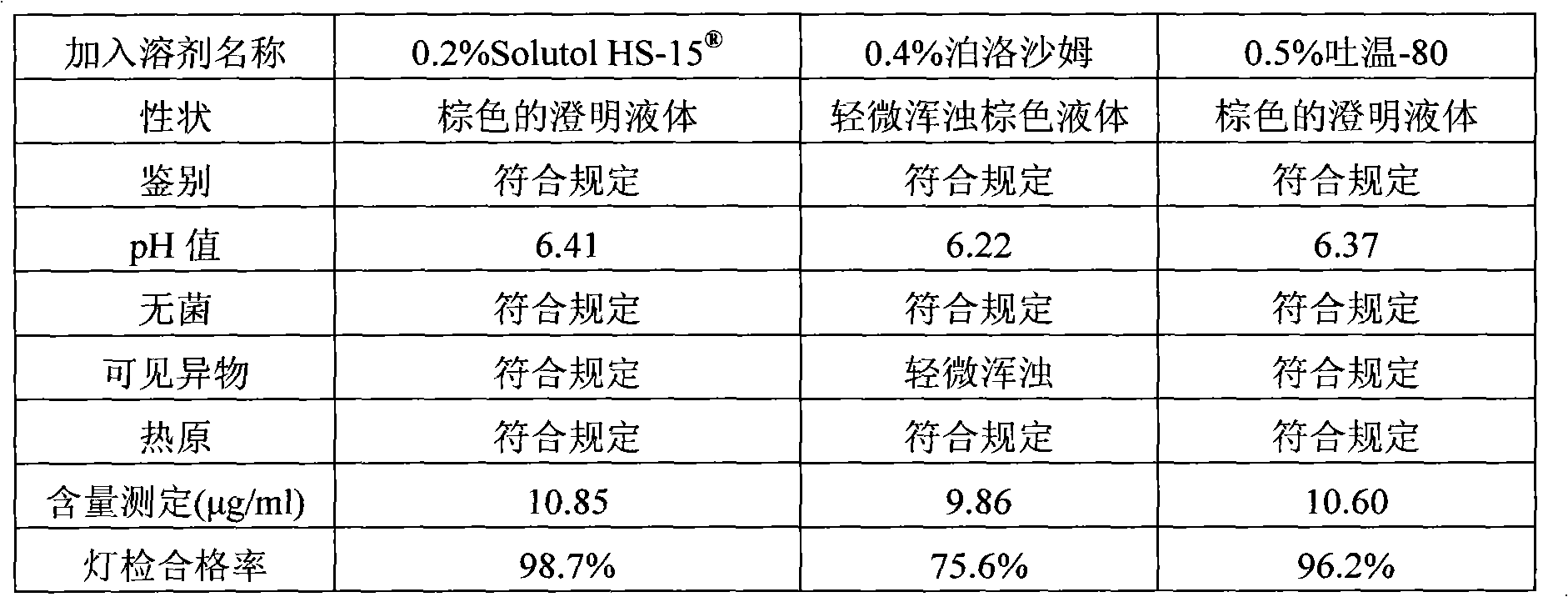
[0036] Polysorbate 80 with Solutol HS- Comparative Test of Stability of Solubilized Compound Angelica Injection
[0037] The detection of visible foreign matter of the compound angelica injection prepared by the present invention complies with the provisions of the drug quality standard and the solution stability is good, which solves the problem that small white spots and white lumps are easy to appear in the compound angelica injection during storage and high-temperature sterilization , solution turbidity and other problems. Utilize the compound angelica injection that the present invention makes according to the relevant requirements of Chinese Pharmacopoeia 2005 edition two appendices XI XC pharmaceutical preparation stability test guiding principle, have investigated respectively placing 36 months at 25 ℃, 6 months at 40 ℃, The stability of the drug was placed at 60°C for 20 days. As a result, the quality of the product was stable under the above test conditions, and al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com