Polymer micelle lyophilized agent encapsulating insoluble antitumor drug
An anti-tumor drug and freeze-dried preparation technology is applied in the field of polymer micelle freeze-dried preparation and preparation of encapsulating taxane-type insoluble anti-tumor drugs, and can solve the problem of affecting the efficacy and safety of the drug and affecting the diffusion of drug molecules. , affecting the anti-tumor effect and other issues, to achieve the effect of facilitating clinical medication, improving therapeutic effect, and improving safety and effectiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Implementation example 1 encapsulates the polymer micelle formulation design of paclitaxel (docetaxel)
[0050] Table 1 is the ratio (w / w%) of each component in the polymer micelle formulation loaded with paclitaxel (docetaxel).
[0051] Table 1
[0052]
[0053] According to prescriptions 1-20 shown in Table 1, paclitaxel (docetaxel) micellar solution can be prepared by film hydration method. Polymer lyophilized formulations.
Embodiment 2
[0054] Example 2 Preparation of polymer micelles freeze-dried formulations loaded with paclitaxel
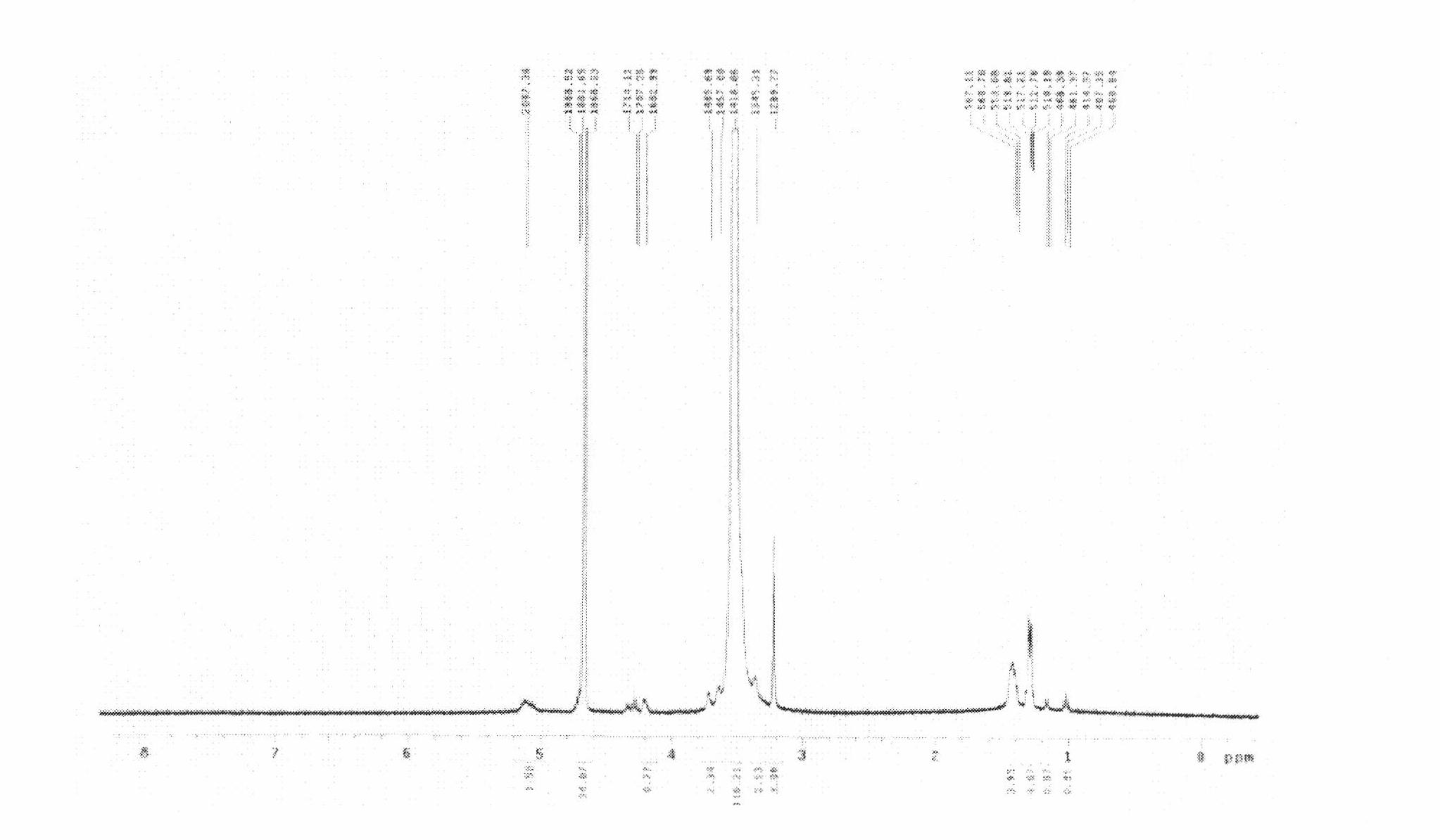
[0055] Take by weighing 0.3g paclitaxel and 2.5g mPEG respectively according to prescription 7 in embodiment 1 2000 -PLA (calculated molecular weight by NMR measurement is 3389) was placed in a 500ml round bottom flask, 100ml of acetonitrile was added, stirred until dissolved, at 50°C, the acetonitrile was evaporated using a rotary evaporator to obtain a transparent gel-like drug film. At room temperature, place in a vacuum oven and dry overnight. Add 50 ml of phosphate buffer (20 mM) at pH 7.0 at 50° C. to dissolve and disperse the drug film to obtain a polymer micelle solution with light blue opalescence. After the solution is filtered and sterilized through a 0.22um sterile filter membrane, it is subpackaged and freeze-dried to obtain a polymer freeze-dried preparation loaded with paclitaxel.
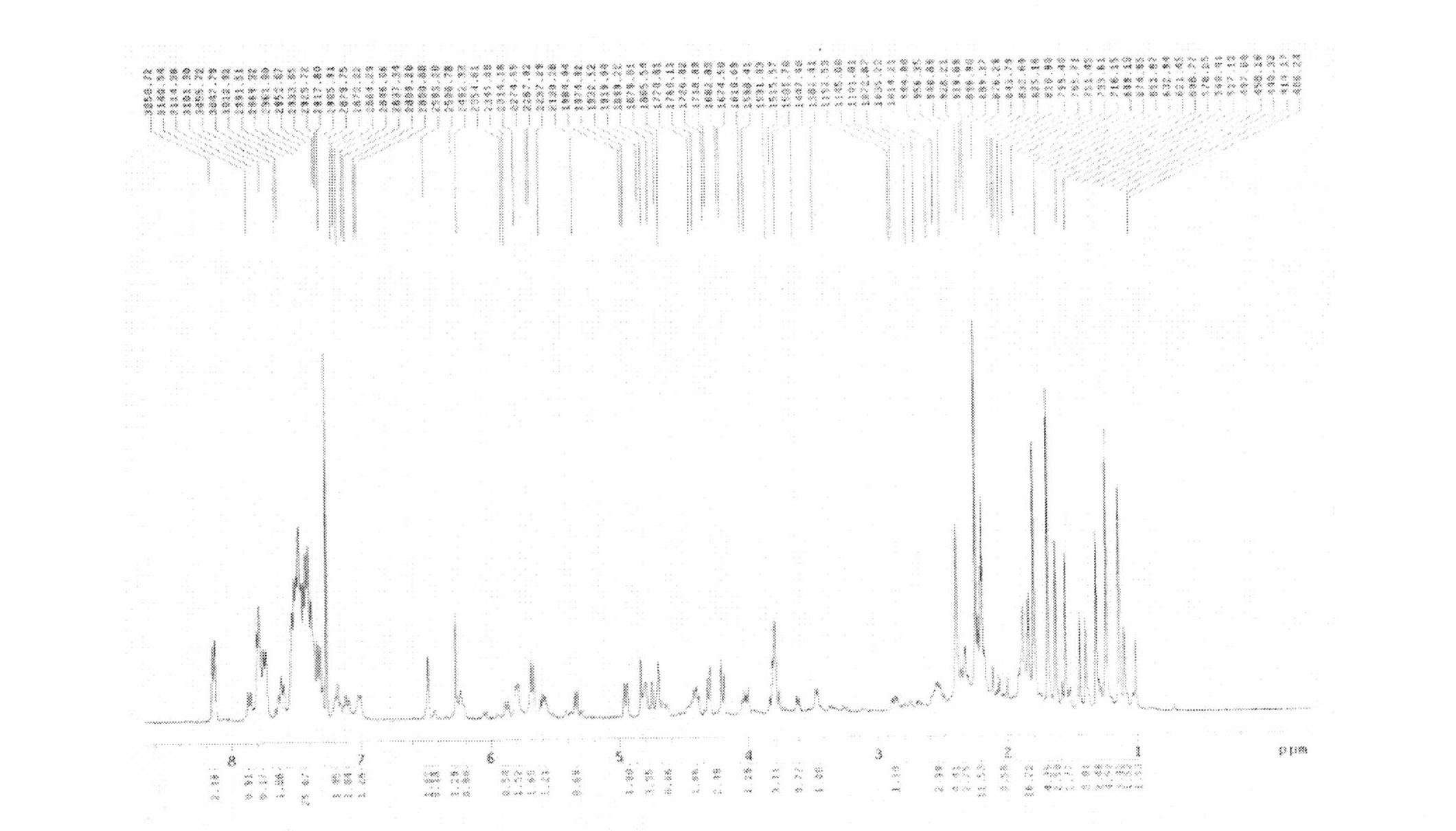
[0056] Such as figure 1 , 2 As shown in , 3, take a small amount of dry powder...
Embodiment 3
[0060] Example 3, preparation of polymer micelles freeze-dried preparations loaded with docetaxel
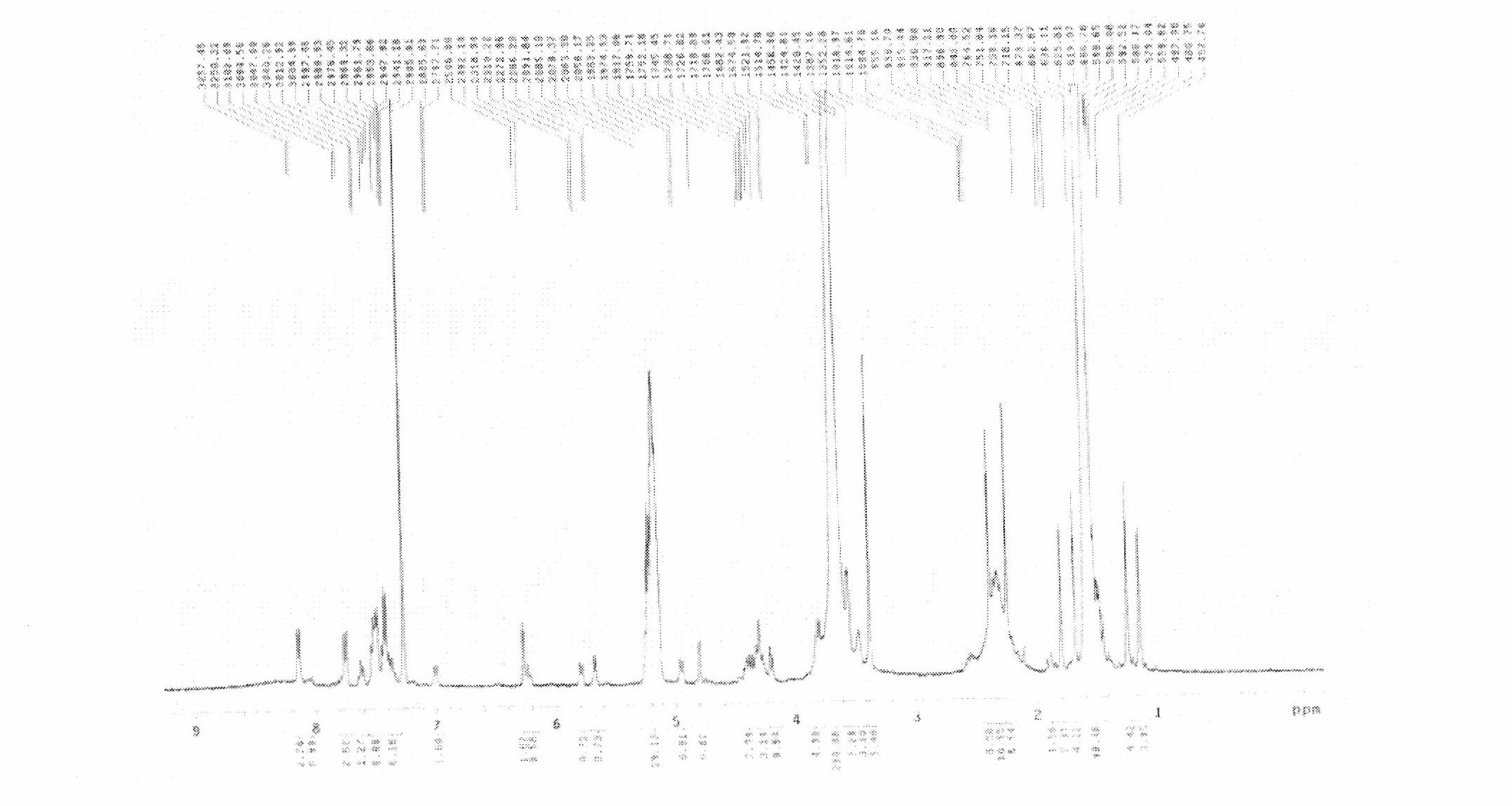
[0061] Weigh respectively 0.60g of docetaxel and 2.4g of mPEG-PLA (the calculated molecular weight is 3778 as determined by NMR) according to prescription 14 in Example 1 and place them in a 1000ml round bottom flask, add 300ml of acetone, stir until dissolved, and at 60°C, use Evaporate acetone with a rotary evaporator to obtain a transparent gel-like drug film; place it in a vacuum oven at room temperature and dry overnight; add 120ml of triple-distilled water at 60°C to dissolve and disperse the drug film to obtain a light blue opalescent film Add 2.4g of lactose as a lyoprotectant to the polymer micelle solution, mix evenly, filter and sterilize through a 0.22um sterile filter membrane, sub-package and freeze-dry to prepare a docetaxel-loaded polymer gel dry preparation.
[0062] The encapsulation rate was determined by the same method as in Example 1 to be 98.2%; the parti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
| refractive index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com