Injection medicinal composition of houttuyfonate
A technology for houttuyniacin and injection, applied in the field of medicine, can solve the problems such as no better solution, unqualified inspection items for visible foreign bodies, easy precipitation of small white spots, etc. Effects of pH stabilization and reduction of Houttuyniacin-degrading substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for preparing a pharmaceutical composition for injection that improves the stability of houttuybein drug injection preparations, comprising the following steps: (1) weighing 0.1 g to 100 g of the raw material medicine calculated as houttuyfonate, and 9.0 g of sodium metabisulfite , citric acid 1mg~2.0g, sodium citrate 1mg~2.0g; (2) citric acid and sodium citrate were prepared into 10%~20% solutions respectively, and set aside. (3) Add to 500ml of water for injection below 40°C, stir until completely dissolved, then add 0.02% (g / ml) activated carbon, stir for 15 minutes, filter and decarburize. (4) Use citric acid or sodium citrate solution to adjust the pH of the filtrate to 3.0-7.0, add water for injection below 40°C to 1000ml; (5) filter the medicinal solution until it is clear, fill it, and sterilize it.
[0027] The specific components and contents thereof of the present embodiment are as follows:
[0028]
[0029] Citric acid and sodium citrate were pr...
Embodiment 2
[0031] Alternatively, the above-mentioned injection pharmaceutical composition for improving the stability of Houttuyfonate injection preparations is prepared according to the following steps:
[0032] (1) Weigh 0.1g-100g of the raw material medicine, 1mg-2.0g of citric acid, 1mg-2.0g of sodium citrate; (2) prepare citric acid and sodium citrate respectively 10% to 20% solution for later use. (3) Add to 500ml of water for injection below 40°C, stir until completely dissolved, then add 0.02% (g / ml) activated carbon, stir for 15 minutes, filter and decarburize. (4) Use citric acid or sodium citrate solution to adjust the pH of the filtrate to 3.0-7.0, add water for injection below 40°C to 1000ml; (5) filter the medicinal solution until it is clear, fill it, and sterilize it.
[0033] The specific components and contents thereof of the present embodiment are as follows:
[0034] Houttuyfonate Hydrochloride 20g
[0035] Citric acid 1.0g
[0036] Sodium citrate 2.0g
[0037] C...
Embodiment 3
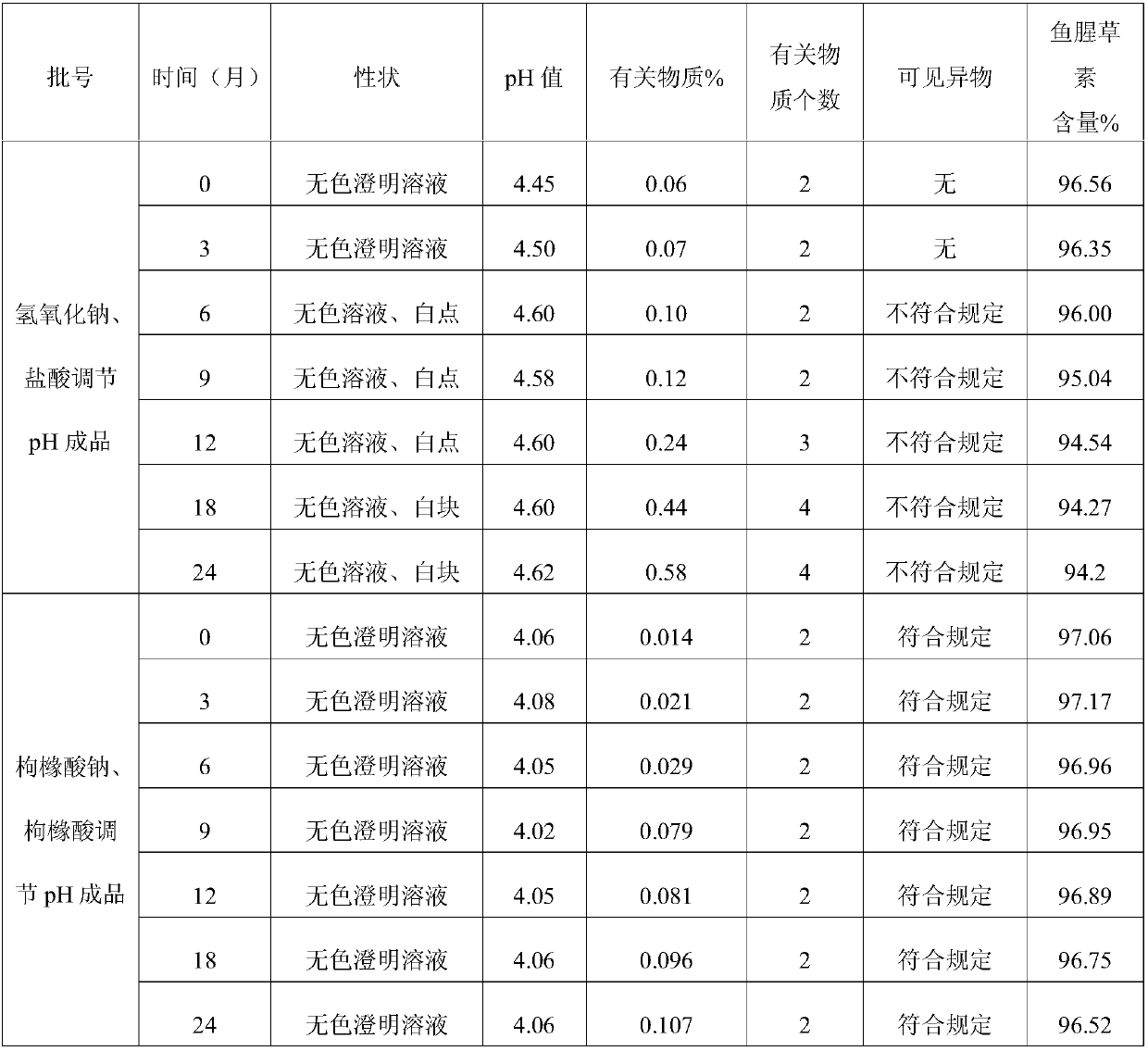
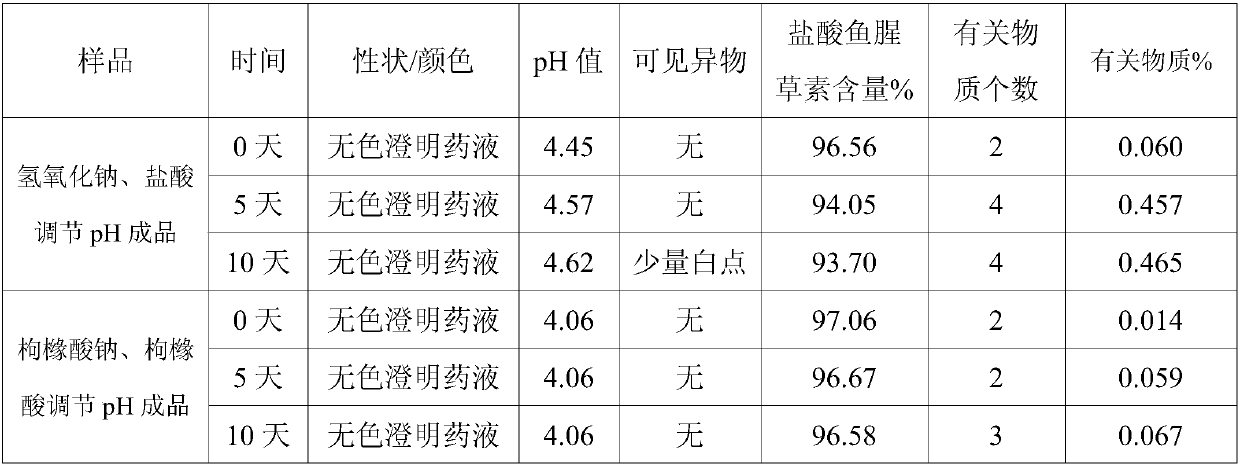
[0039] Comparative test on the stability of Houttuydin Sodium Metabisulfite Injection
[0040] The detection of visible foreign matter in Houttuyfonate Sodium Metabisulfite Injection prepared by the present invention complies with the provisions of the drug quality standard, and the stability of the solution is very good. In the case of avoiding the use of other co-solvents that increase the risk of clinical application, it solves the problem of fish During the storage process, the Houttuybein Sodium Metabisulfite Injection is prone to problems such as small white spots, white lumps, and cloudy solution. Utilize the Houttuyfonate Sodium Metabisulfite Injection prepared by the present invention according to the relevant requirements of the Chinese Pharmacopoeia 2005 Edition Two Appendix XIXC Drug Preparation Stability Test Guiding Principles, investigated respectively placed at 25 ℃ for 24 months, 40 ℃ for 6 months months, 10 days at 60°C, and 20 days at 0-5°C for 20 days. As a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com