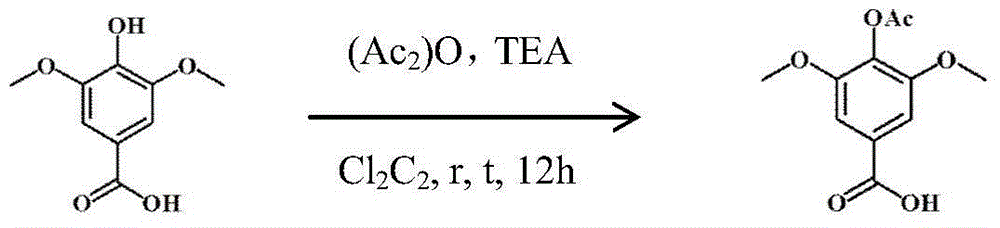Patents
Literature
78 results about "Leonurine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
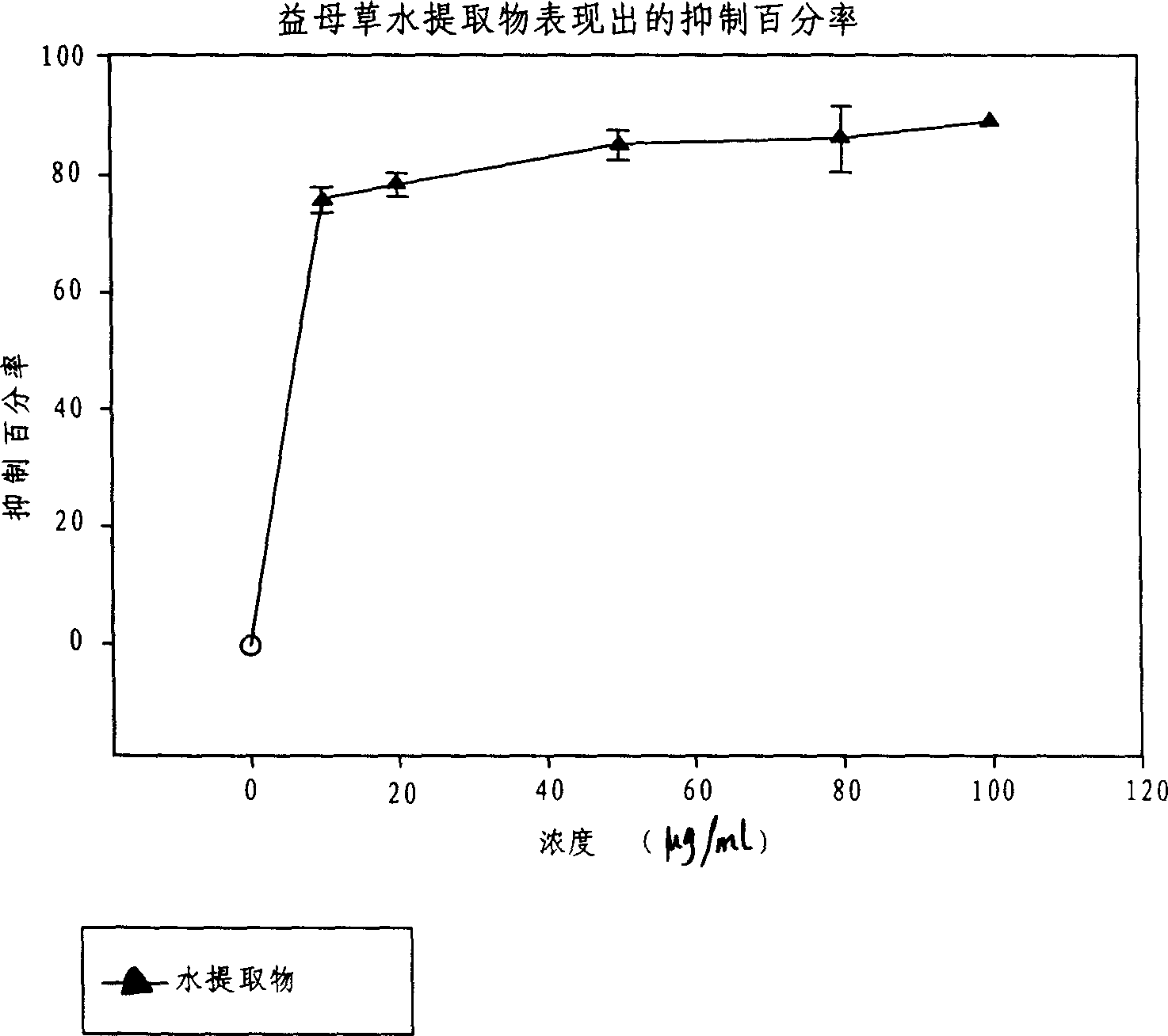
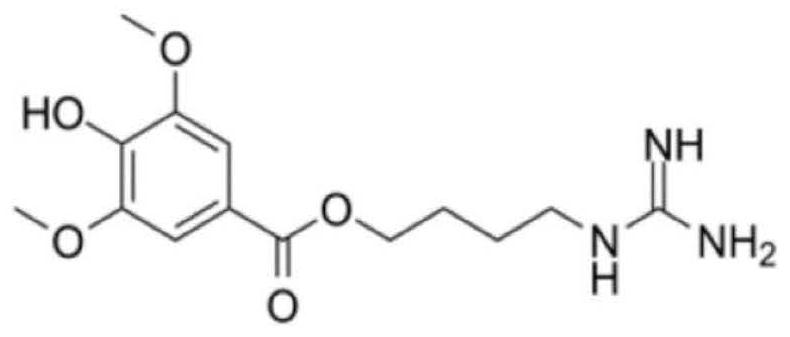
Leonurine is a pseudoalkaloid that has been isolated from Leonotis leonurus, Leonotis nepetifolia, Leonotis artemisia, Leonurus cardiaca (Motherwort), Leonurus sibiricus, as well as other plants of family Lamiaceae. Leonurine is easily extracted into water.
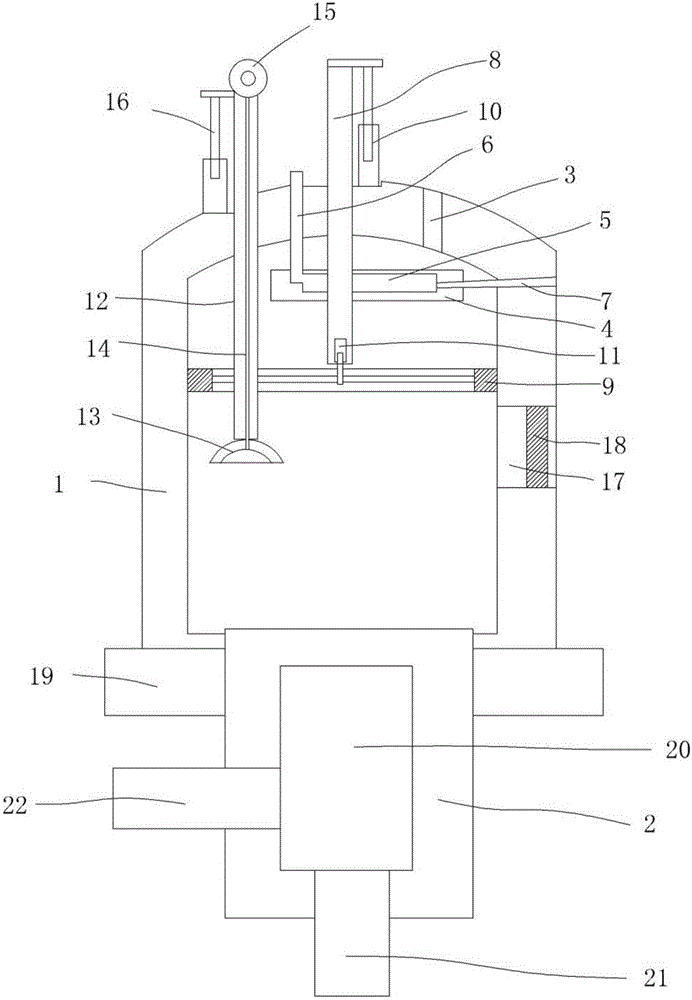
Salt of leonurine and its preparation
InactiveCN1415603ALess irritatingGuaranteed stabilityOrganic active ingredientsOrganic chemistryLeonurineOrganic acid
Owner:李晓祥
Method for synthesizing leonurine
A process for synthesizing the leonurine from syringic acid as initial raw material includes such steps as carbonylation reaction, acylchlorination reaction, esterification reaction, ammonification reaction to obtain leonuriamine, and reacting with methylisothiourea. Its advantages are simple operation, easy control, high output rate, and high purity (99.8%).
Owner:李晓祥
Application of acetylcholine esterase inhibitor medication of Yidancao extractive as cholinomimetic
InactiveCN1915292AEnhance pharmacological effectsRich sourcesNervous disorderDigestive systemDiseaseDepressant
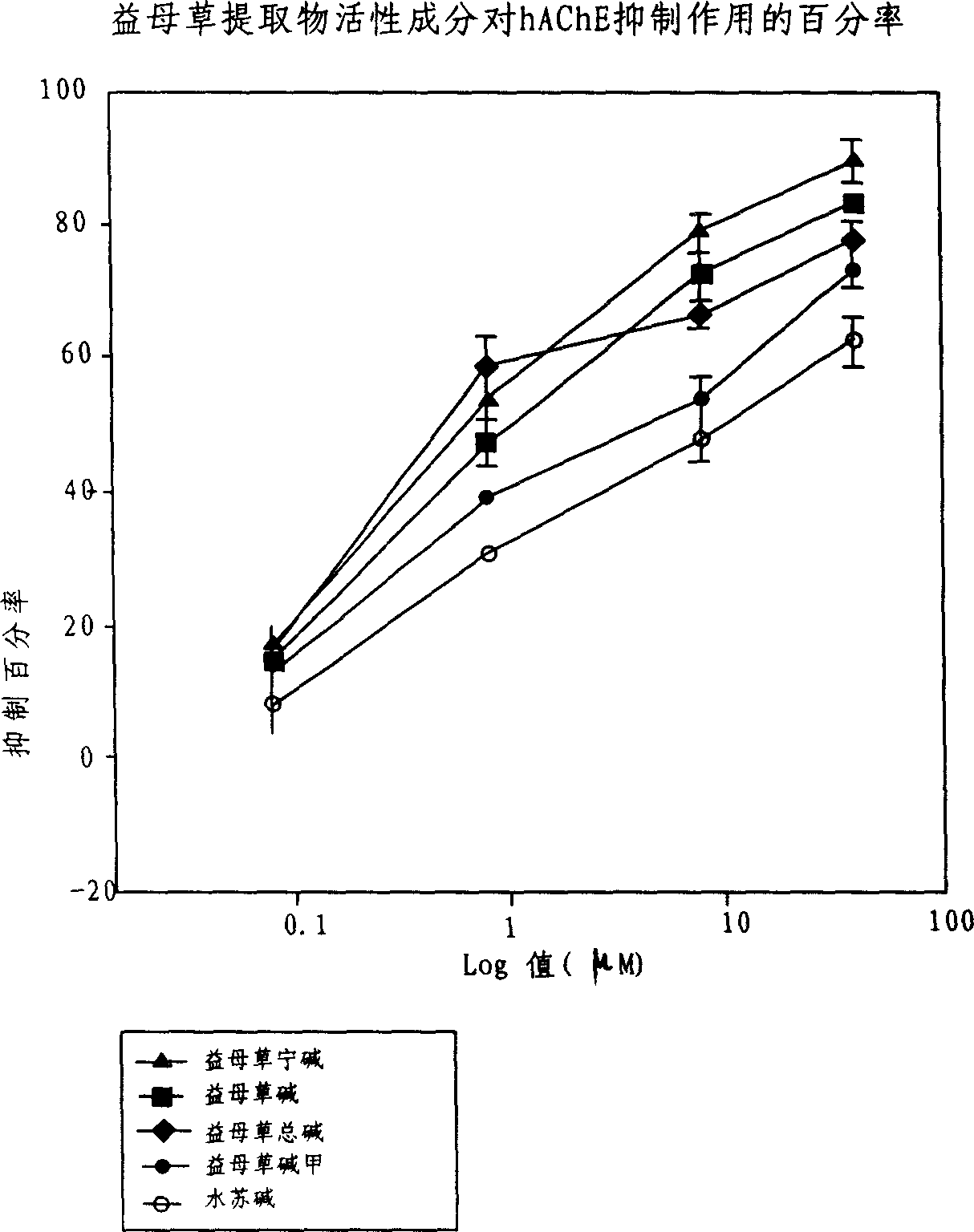
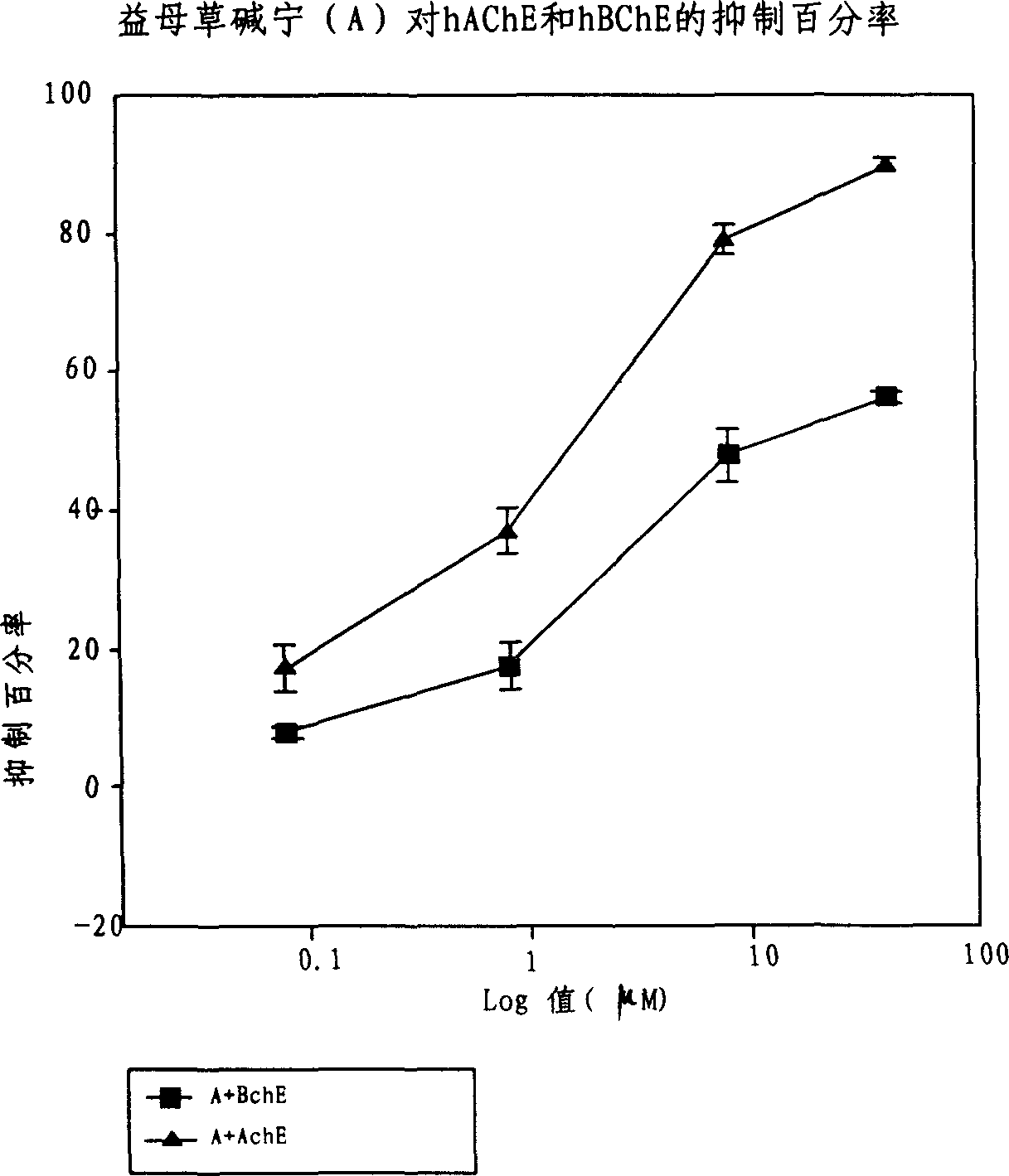
An application of the motherwort's extract including leonurinine, leonurine, general leonurine, leonurine A, cadabine in preparing the medicines as acetylcholinesterase depressant for treating and preventing more than 20 diseases including senile dementia, Parkinson's disease, cardiovascular and cerebrovascular diseases, etc is disclosed.
Owner:郝书平
Process for extracting leonuridine from fresh motherwort and leonuridine preparation
A process for extracting leonurine from fresh motherwort includes such steps as extracting juice, depositing in alcohol, filtering, column exchanging, eluting, concentrating and drying. Said leonurine can be made into capsule, tablet, or injection for treating gynopathy with hige curative effect.
Owner:ZHEJIANG DADE PHARMACEUTICAL GROUP CO LTD
Applications of leonurine in preparation of medicines used for treating atherosclerosis
InactiveCN103565786AImprove integrityReduce depositionOrganic active ingredientsCardiovascular disorderRabbit modelAtheroma
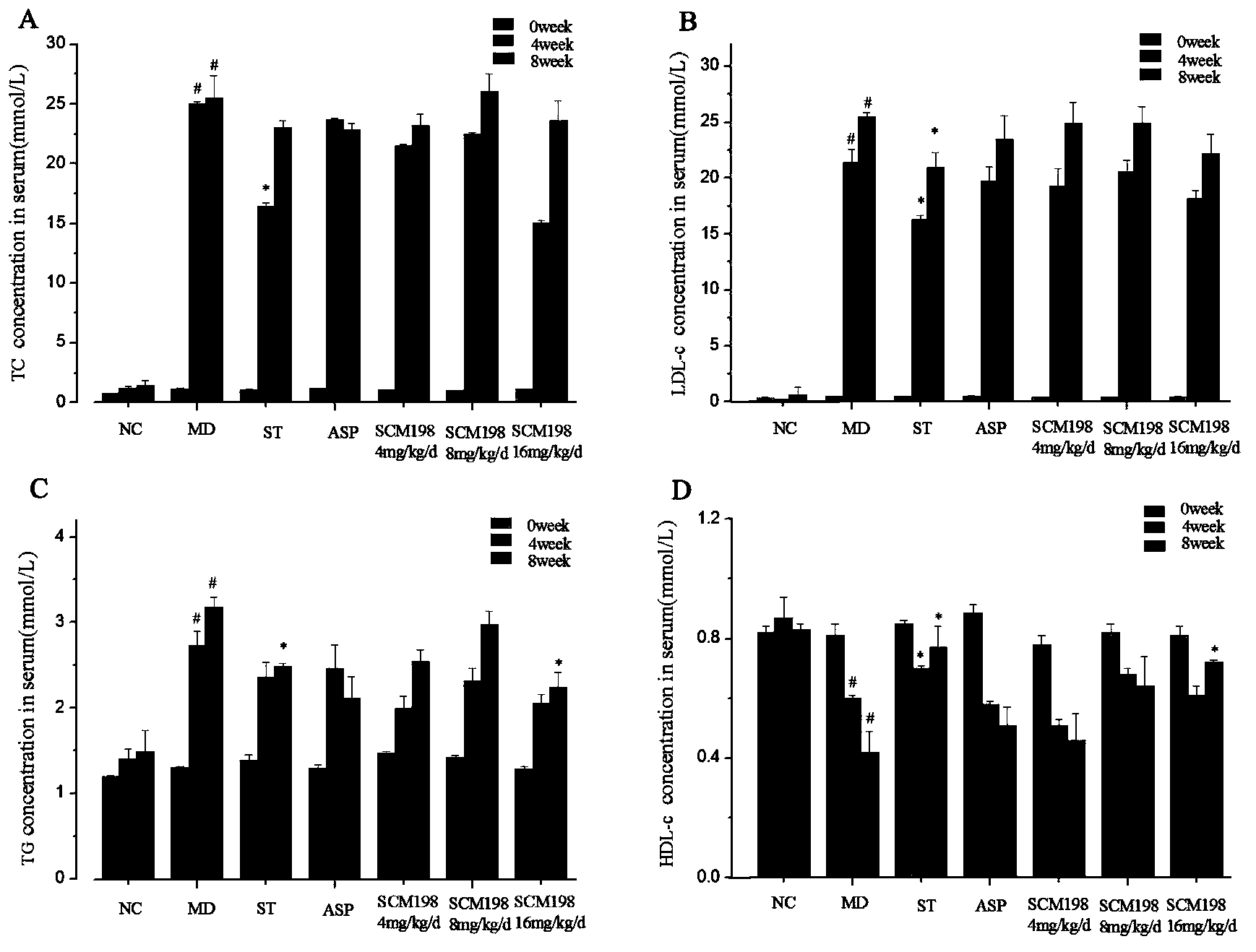
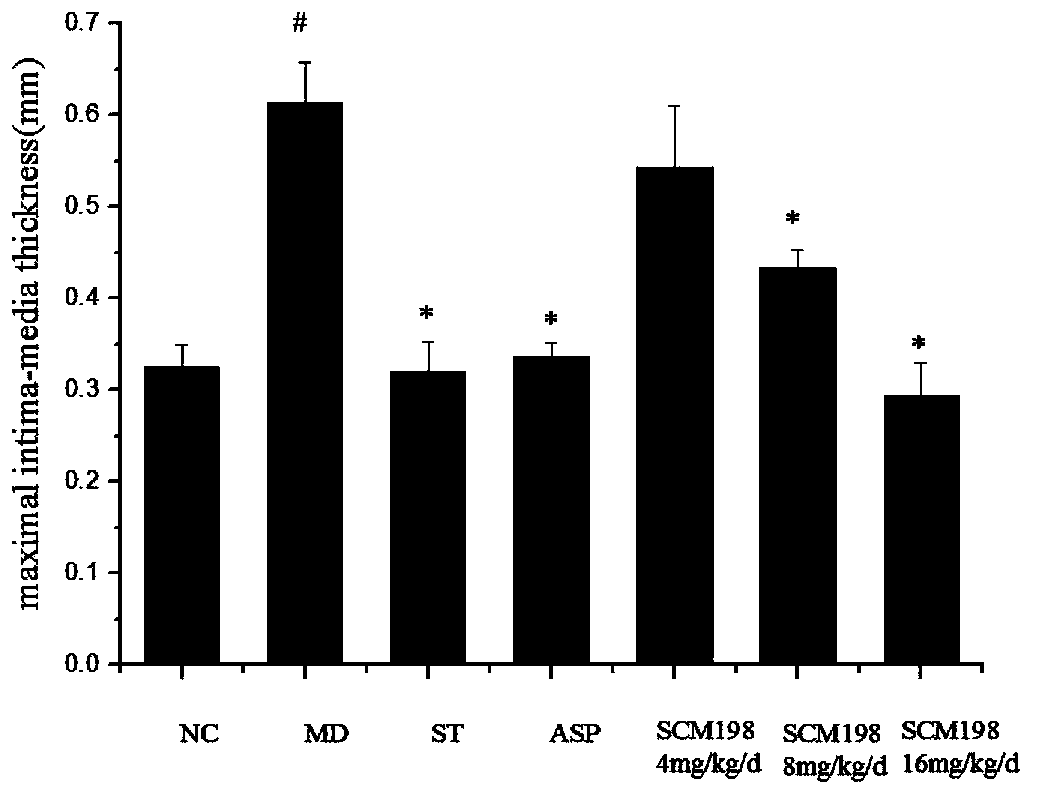
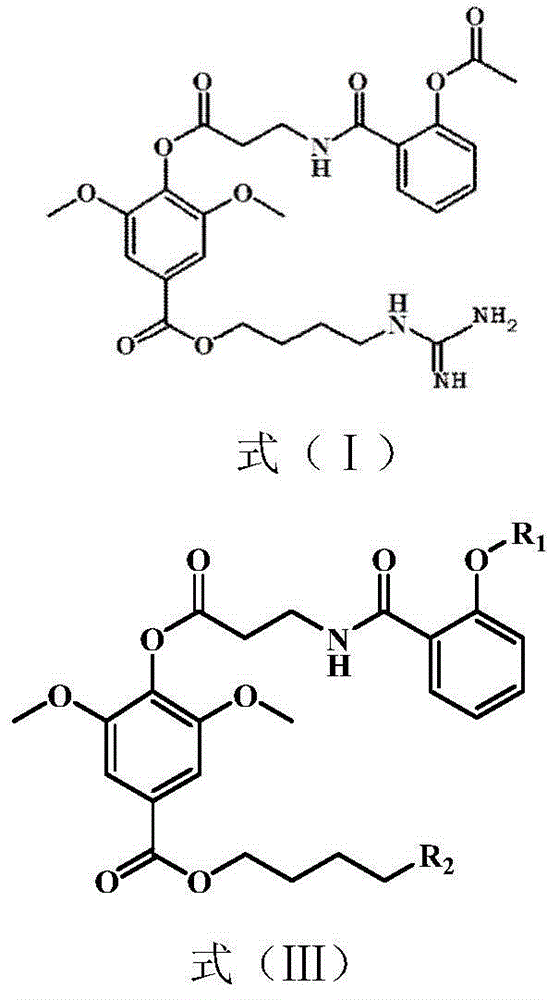
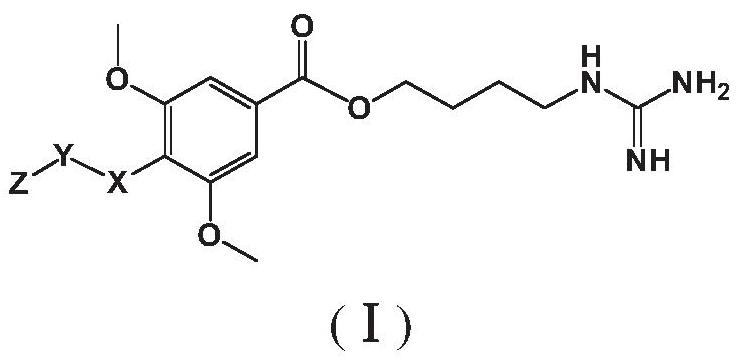
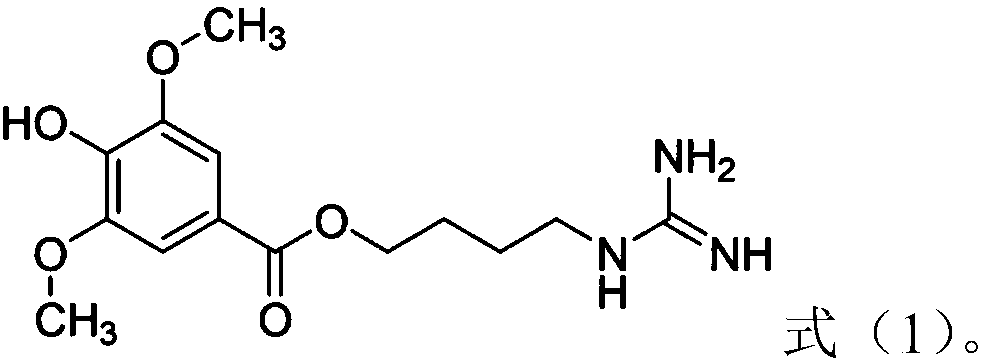
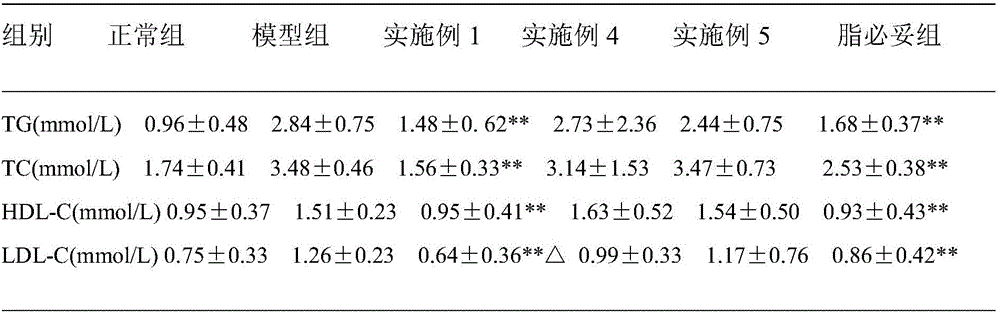
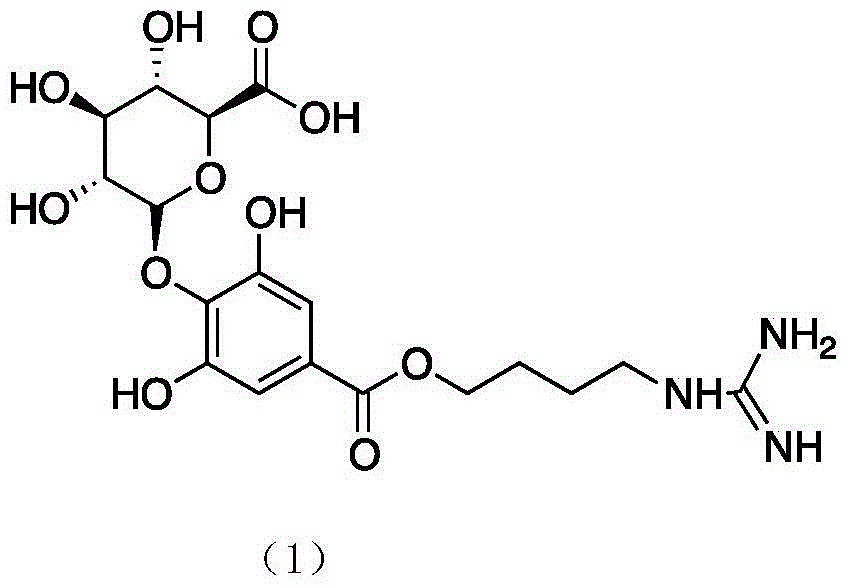
The invention belongs to the field of pharmacy, and relates to applications of leonurine in preparation of medicines used for treating atherosclerosis. Leonurine possesses a structure represented by formula (I). It is shown by results of experiments on a rabbit model with high fat diet induced atherosclerosis that, leonurine possesses anti-inflammatory activity and antioxidant activity, so that leonurine is capable of increasing the content of high-density lipoprotein, reducing the content of total cholesterol, reducing mean blood flow velocity of arteries, improving blood flowing states, protecting the integrity of blood vessel endothelium, and reducing deposition of lipid in blood vessels and generation of macrophage, and can be used for treating atherosclerosis.
Owner:FUDAN UNIV
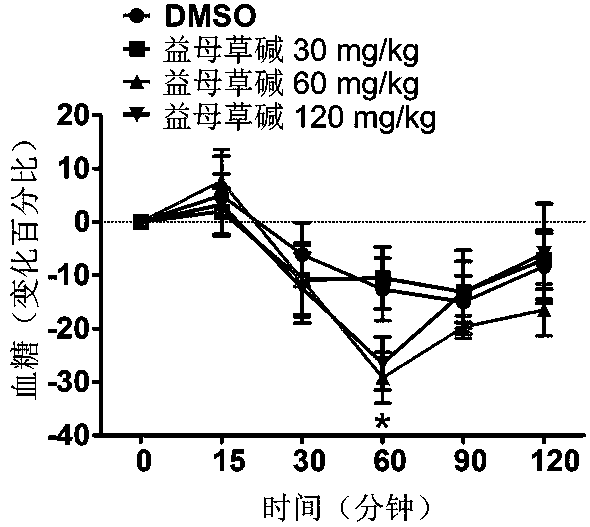
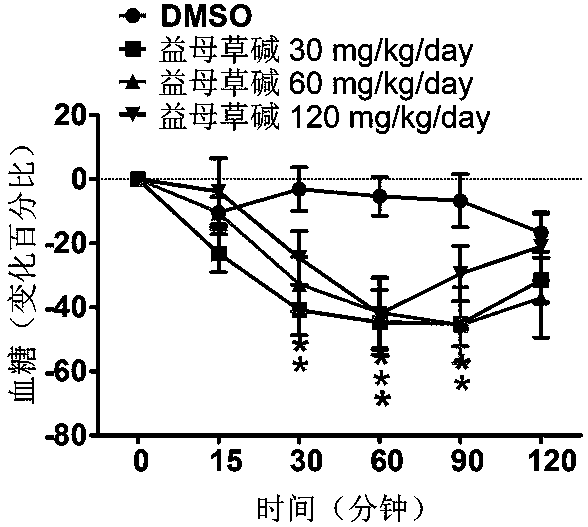
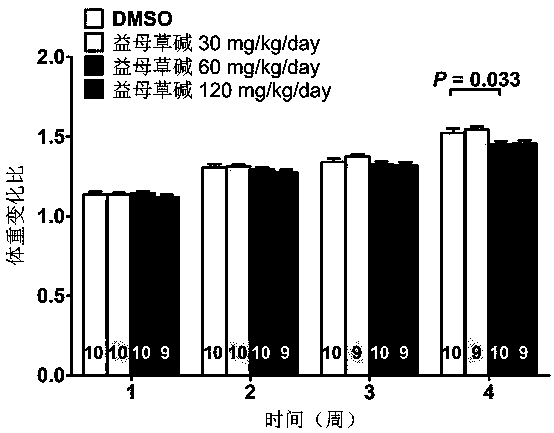
Application of leonurine to preparation of medicament for treating 2-type diabetes
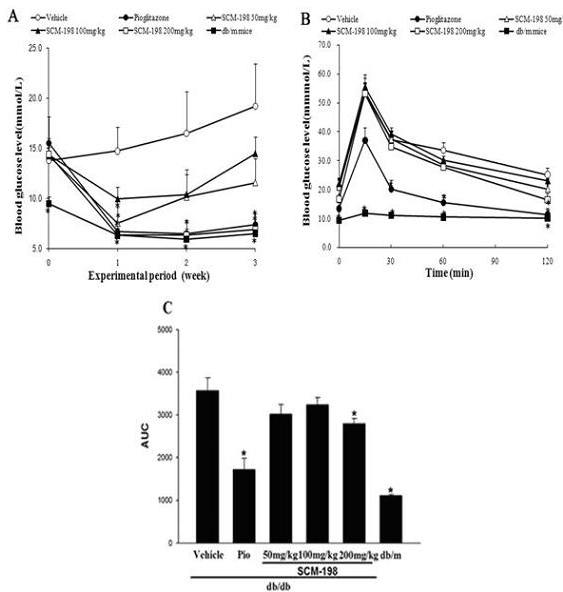
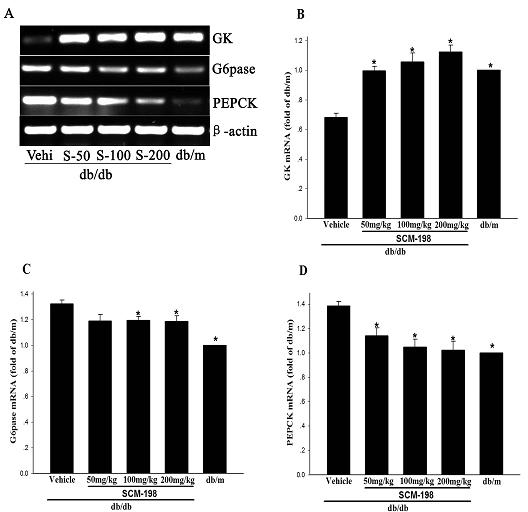
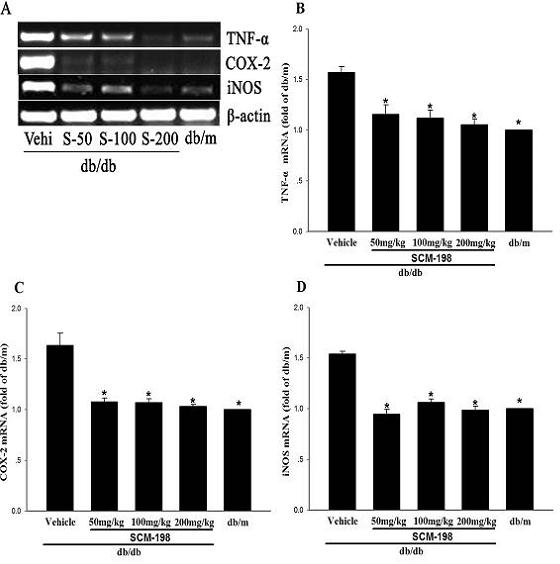
The invention belongs to the field of traditional Chinese medicine manufacturing, and relates to application of leonurine to preparation of a medicament for treating 2-type diabetes. Animal experiments prove that the fasting blood glucose of a 2-type diabetes mouse, i.e., a db / db mouse, can be lowered by the leonurine, and the tolerance of oral glucose is improved; and meanwhile, fasting plasma insulin is increased, the plasma triglyceride is reduced and the content of the plasma high-density lipoprotein is increased. Experiment results also show that the expression of liver glucose metabolic enzymes such as glucokinase, glucose-6-phosphatase and phosphoenolpyruvate carboxyl enzyme is adjusted by the leonurine in an Akt dependent mode; and the biological response of an inflammatory mediator, such as generation of TNF (Tumor Necrosis Factor)-alpha, degradation of IkB-alpha and subsequent phosphorylation of NF-kBp65, is suppressed. Through the leonurine, the inflammatory state of the 2-type diabetes can be corrected, and the symptoms of the 2-type diabetes can be improved; and the leonurine can be used as a treatment medicament to be applied to the treatment of the 2-type diabetes.
Owner:FUDAN UNIV
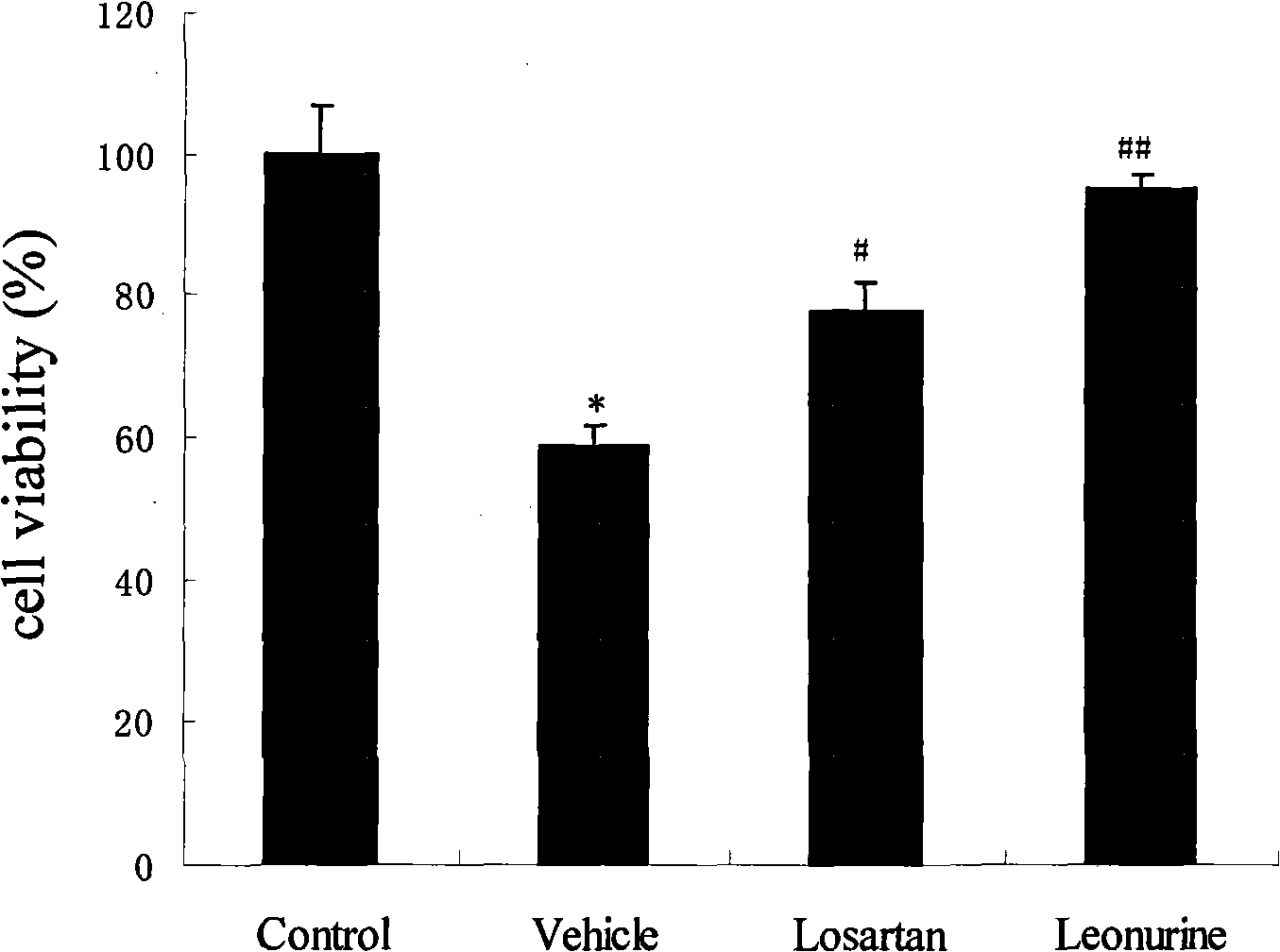
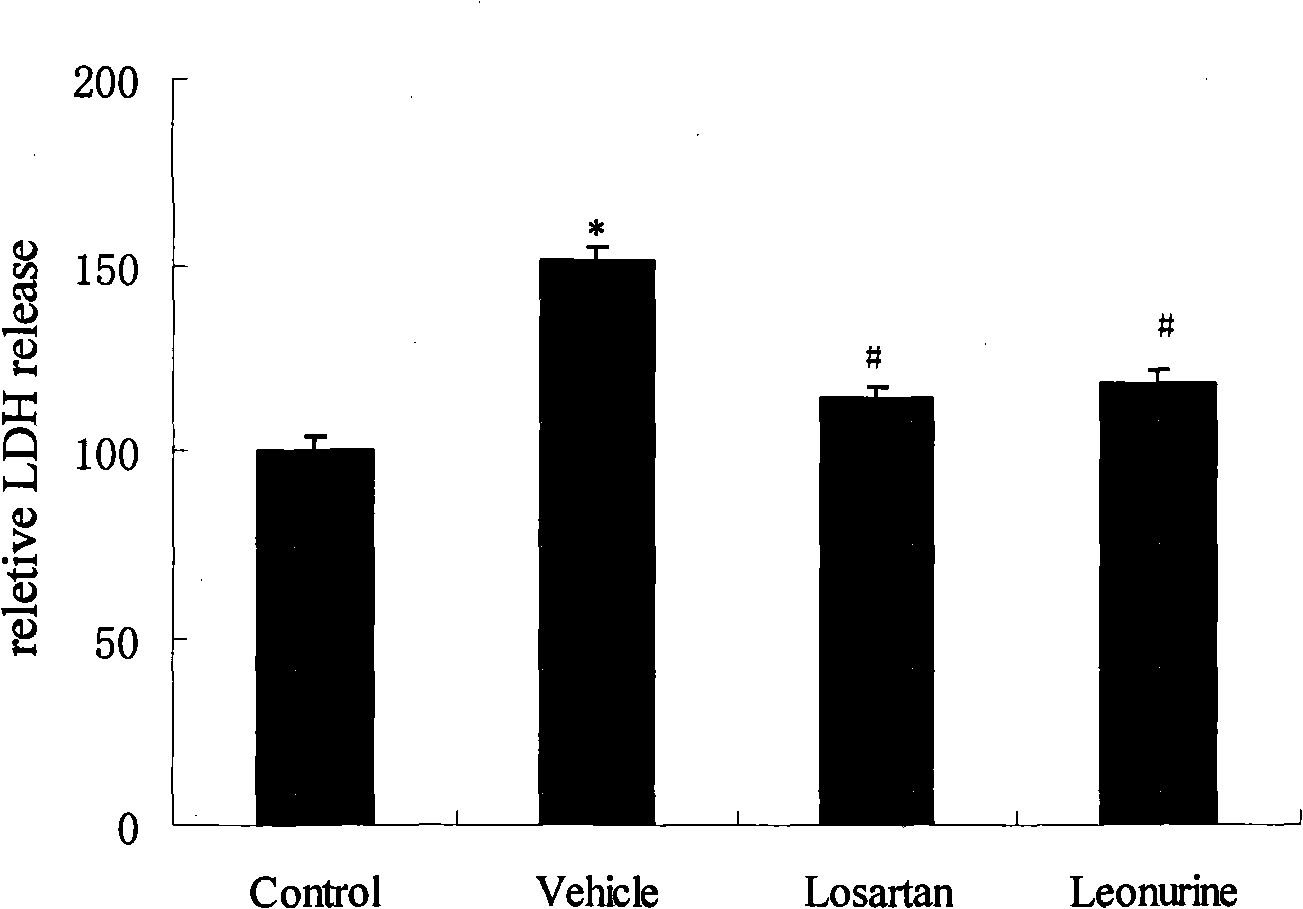
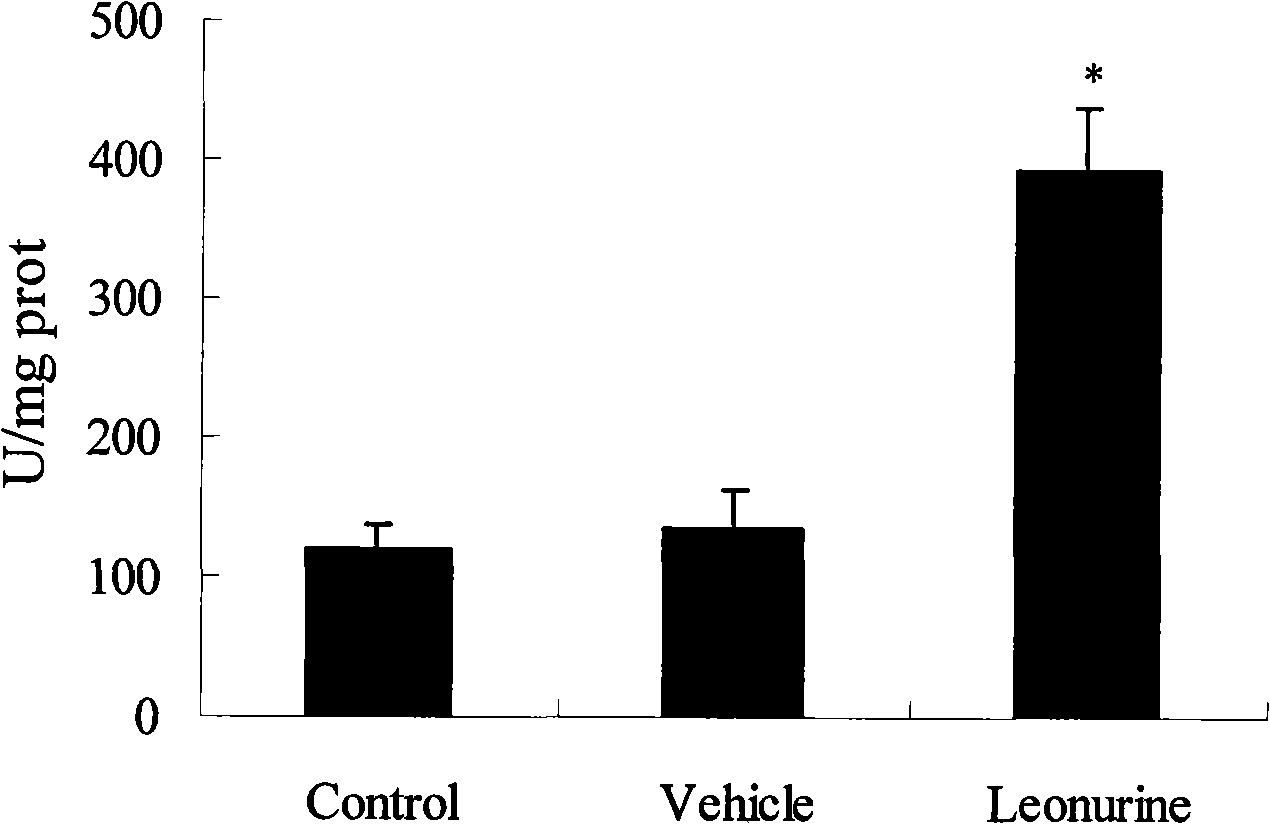
Use of leonurine in preparing medicine for preventing and treating ischemic cardiomyopathy
The invention belongs to the Chinese medicine pharmaceutical field and relates to the application of specific monomer leonurine in Chinese medicinal herb motherwort in preparing medicine combination, in particular to the application in preparing the medicine combination which cures ischemic cardiomyopathy. By the experimental research of anoxic myocardium models in vitro, the result shows that the leonurine can increase livability of ischemic myocyte, can decrease the leakage rate of lactate dehydrogenase, can increase the activity of antioxidase catalase, can increase the activity of SOD, can minish the level of lipid peroxidation products, and proves that the leonurine has obvious protective effect on ischemic myocardium and prepares the medicine which cures ischemic cardiomyopathy.
Owner:ZHUHAI HENGQIN NEW DISTRICT ZHONGZHU ZHENGTAI MEDICAL MANAGEMENT CO LTD
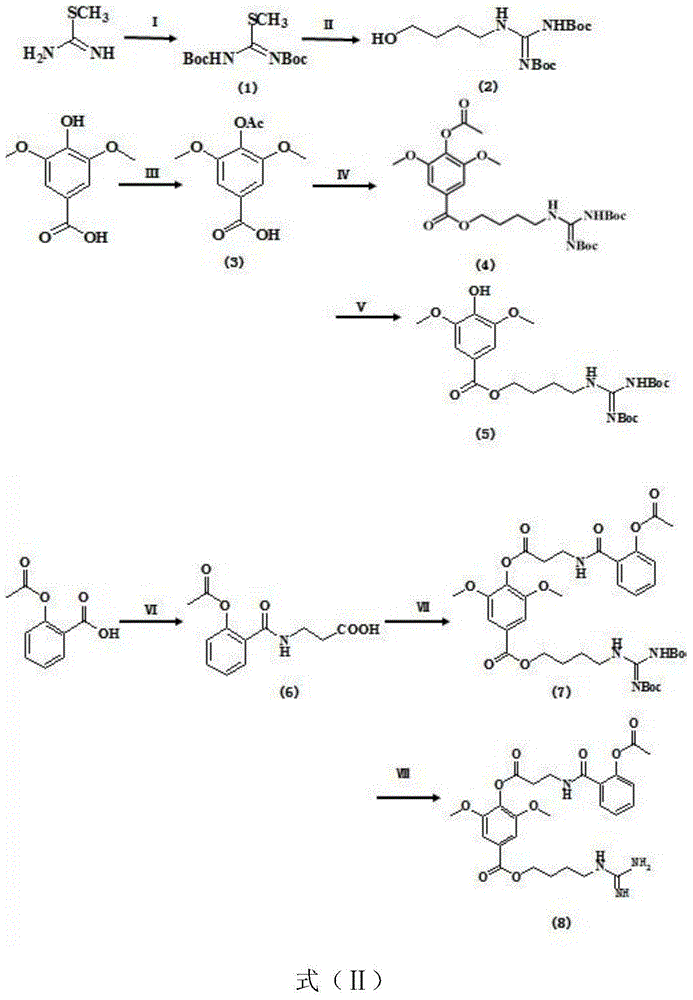
Preparation method of leonurine and aspirin conjugate
InactiveCN106146355AHigh yieldSave raw materialsUrea derivatives preparationOrganic compound preparation4-toluenesulfonic acidO-acetylsalicylic acid
The invention belongs to the field of medicinal chemistry, and relates to a synthesizing method of leonurine and aspirin conjugate. The method comprises the steps that syringic acid, S-methylisothiourea and aspirin serve as starting materials, reactions of acetylation, BOC protection, amidation, deacetylation and the like are conducted, and corresponding structure segments are obtained; dilauryl hydrogen phosphite, 4-dimethylaminopyridine and 4-toluenesulfonic acid are used together to serve as a condensation catalyst to connect the three segments, de-BOC protection is conducted on trifluoroacetic acid, and a target compound is obtained. The synthesizing method of the leonurine and aspirin conjugate is easy and convenient to operate, mild in condition, simple in post treatment and capable of increasing the yield of the intermediate leonurine product; the total yield of the prepared leonurine and aspirin conjugate is 25.27%, a pharmacological activity test is conducted, it is shown through the result that very good antioxidant and anti-apoptosis effects at a cellular level are achieved, and the effect on protecting myocardial cells is exact.
Owner:FUDAN UNIV
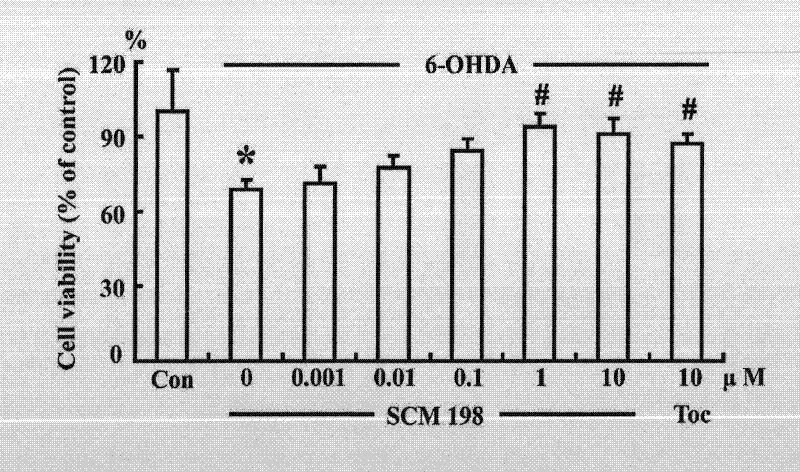
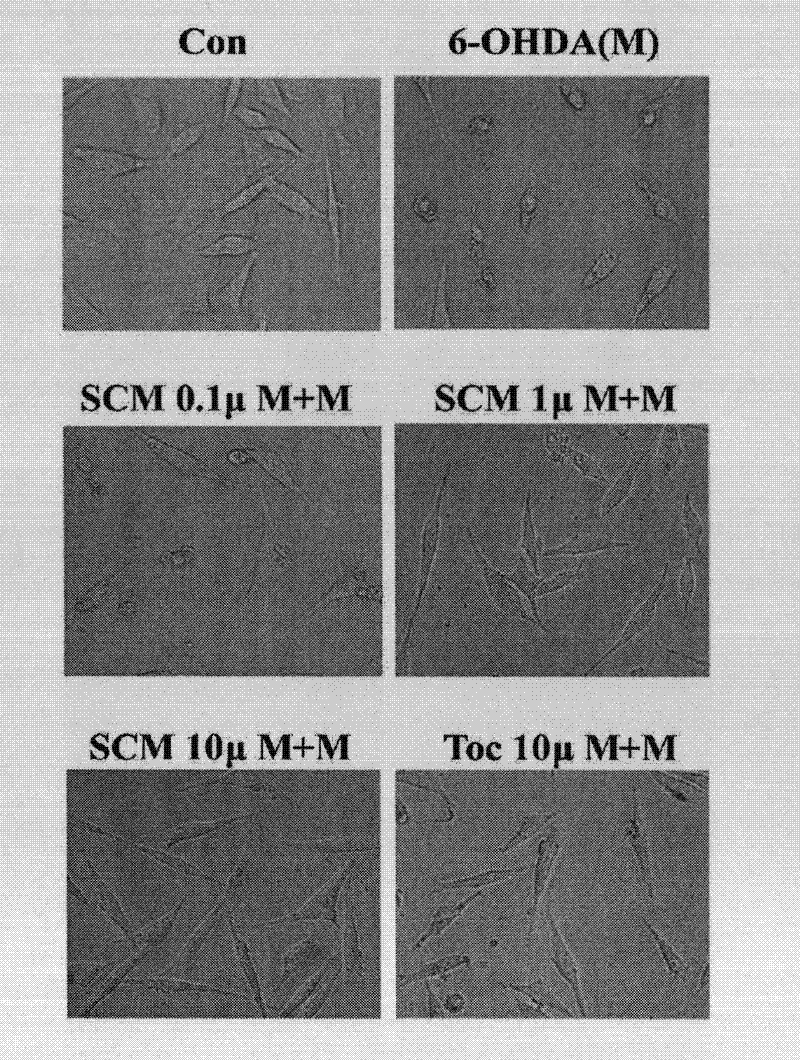
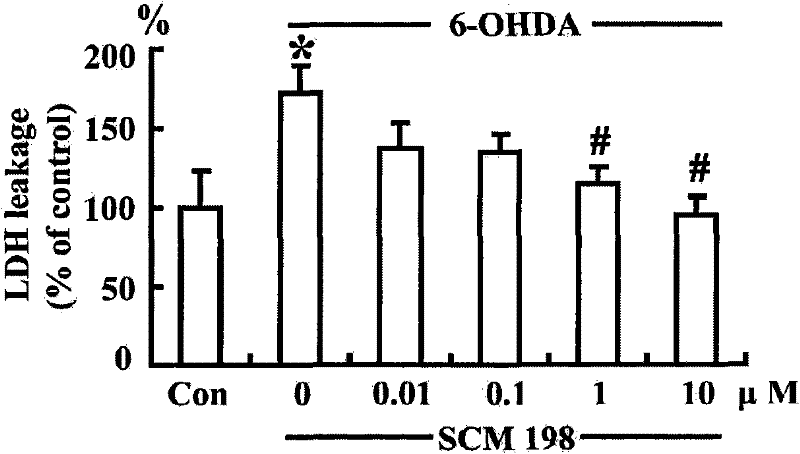
Application of leonurine in preparation of medicines used for preventing and treating parkinson disease
InactiveCN102475699AProtectiveOptimize quantityOrganic active ingredientsNervous disorderSuperoxideAntioxidative enzyme
The invention belongs to the traditional Chinese medicine pharmacy field, and relates to an application of leonurine SCM198 in preparation of medicines used for preventing and treating parkinson disease. According to the invention, neurotoxin 6-OHDA induces damage of nerve cells in rats, the result shows that leonurine SCM198 is capable of improving behavior changes of PD model rats, increasing the survival rate of damaged nerve cells, raising antioxidant enzyme superoxide dismutase activity, minimizing excessive generation of active oxygen species, stabilizing mitochondrial membrane potential, minimizing apoptotic cells and regulating the change level of Bax and Bcl2 gene and protein. The result proves that the leonurine SCM198 has protection effect on a PD model, and can be used for preparing medicines for preventing and treating PD.
Owner:FUDAN UNIV
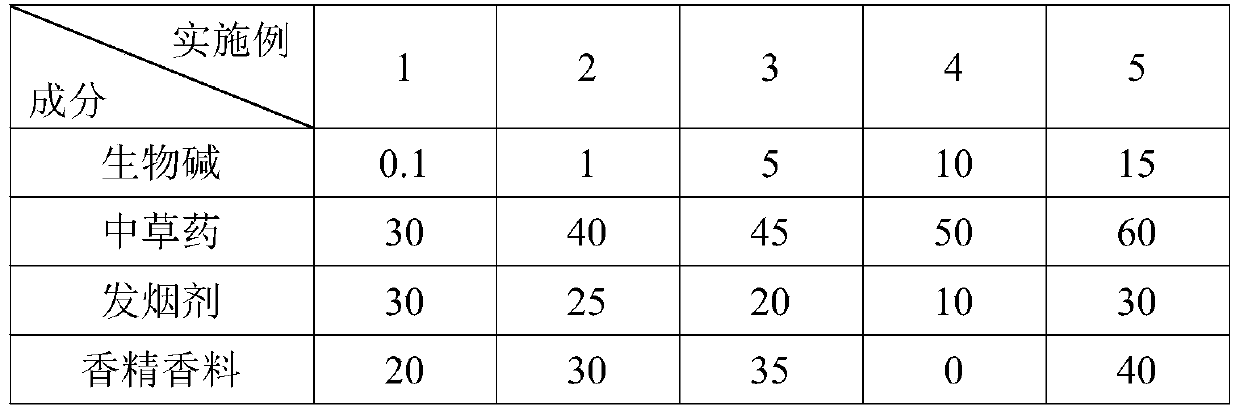
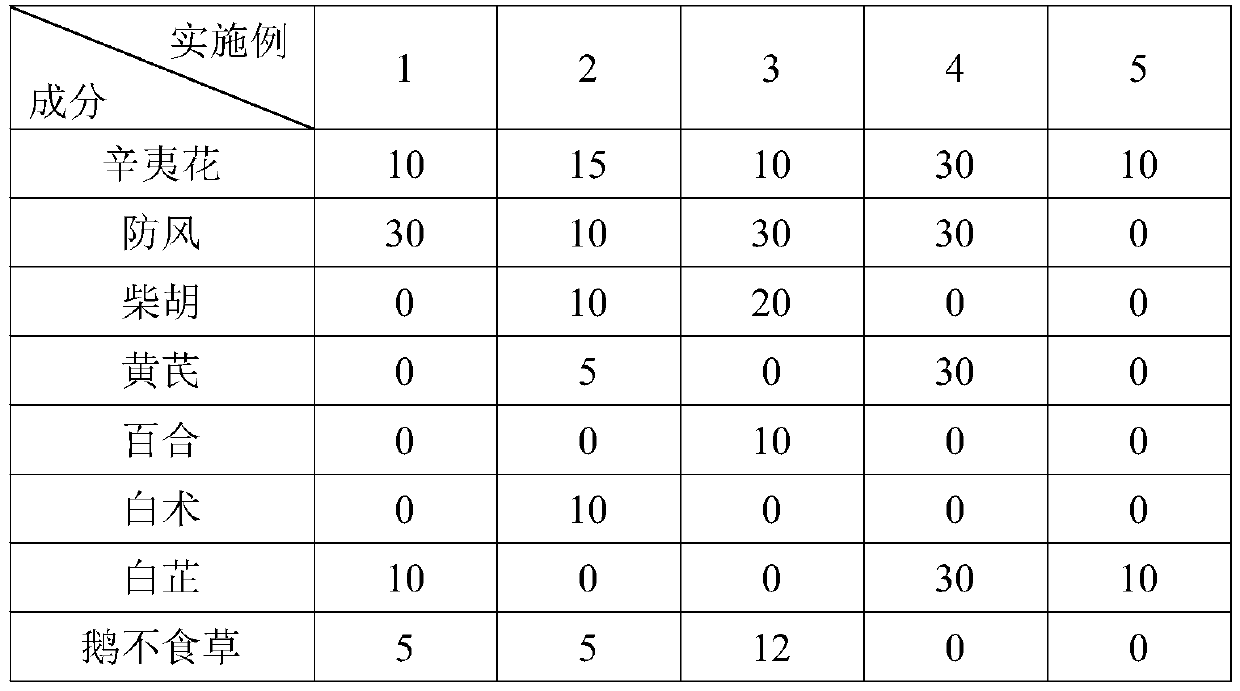
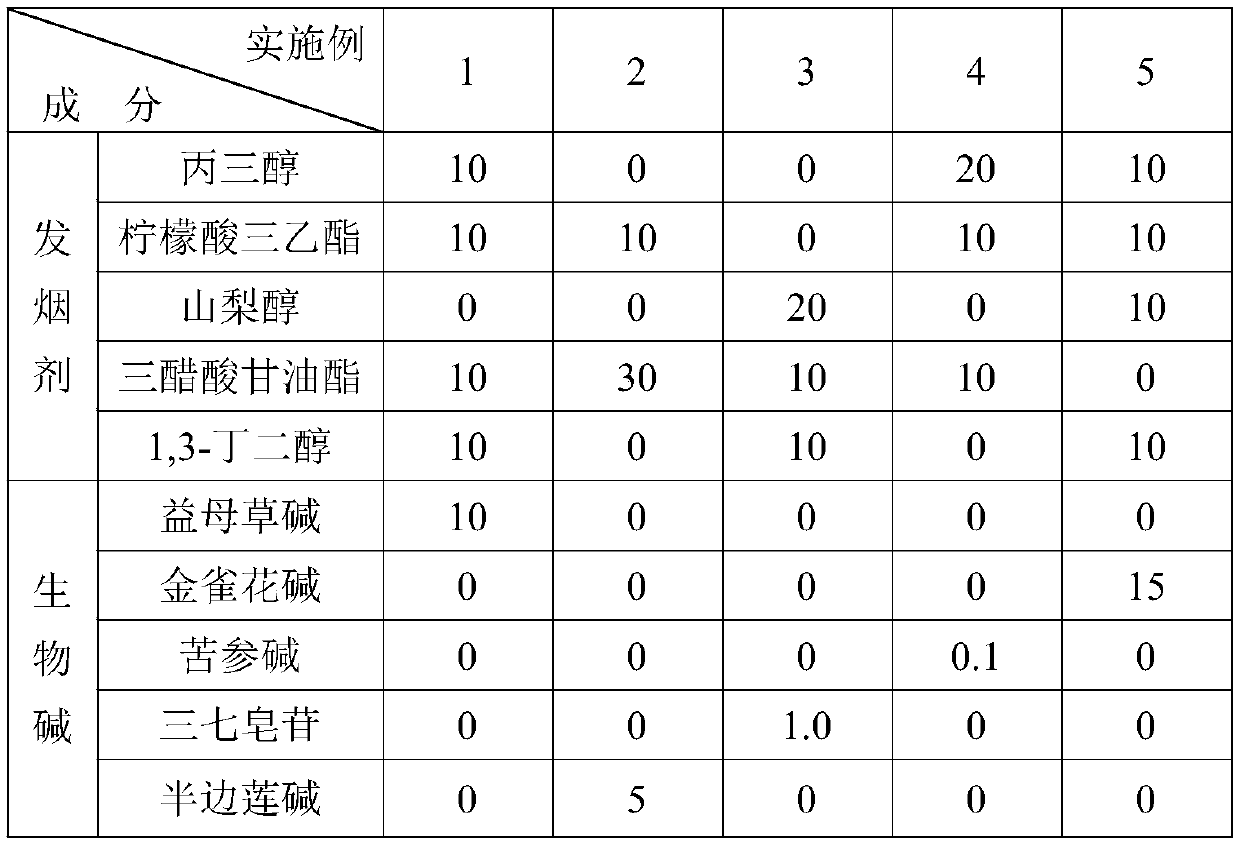
Alkaloid fuming particles for low-temperature heating non-combustible products and a preparation method of alkaloid fuming particles
The invention discloses alkaloid fuming particles for low-temperature heating non-combustible products. The alkaloid fuming particles comprise the following components in parts by weight: 0.1-15 partsof alkaloid, 30-60 parts of Chinese herbal medicines and 10-30 parts of a smoke agent, wherein the alkaloid is any one of cytisine, leonurine, sophocarpidine, notoginsenoside and lobeline. The invention also discloses a preparation method of the alkaloid fuming particles. According to the invention, the fuming particles adopt alkaloid and natural Chinese herbal medicine formulas and can replace nicotine-containing tobacco, so that addiction and health hazards are reduced, and meanwhile, the effects of refreshing, decompressing and medicine health care are achieved.
Owner:YUNNAN XIKE TECH CO LTD
Synthetic method for leonurine
ActiveCN106866464AMild reaction conditionsEasy to controlOrganic compound preparationCarboxylic acid esters preparationSulfateLeonurine
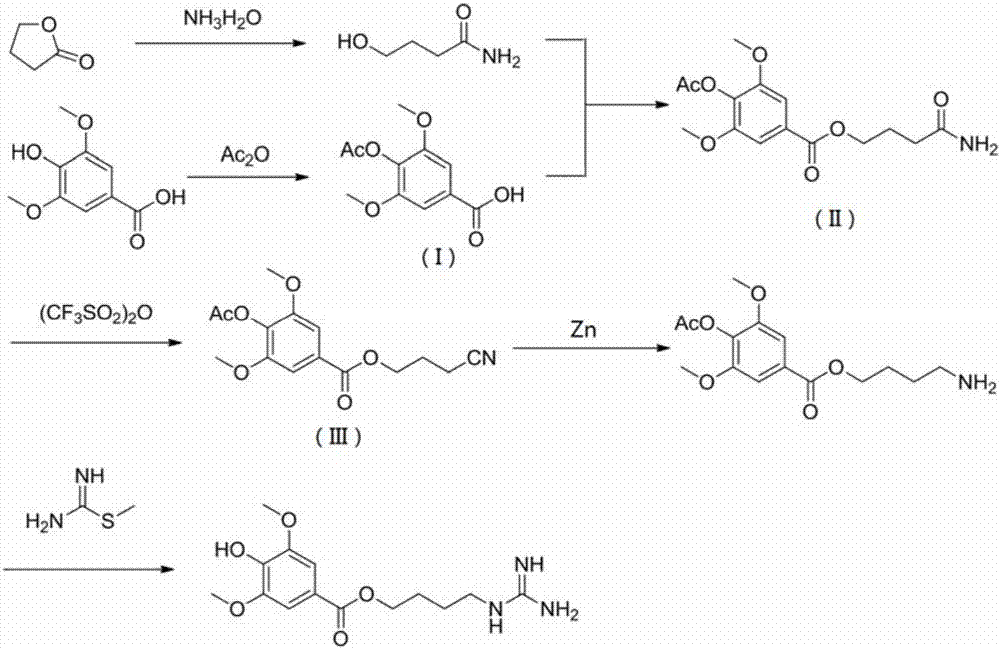
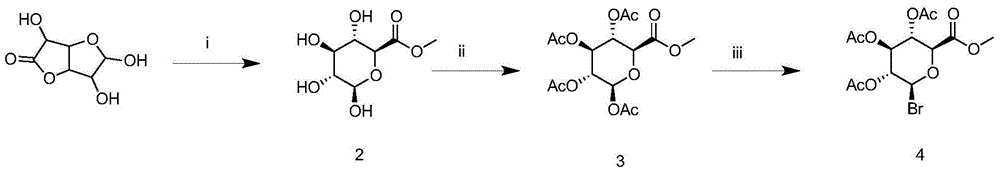
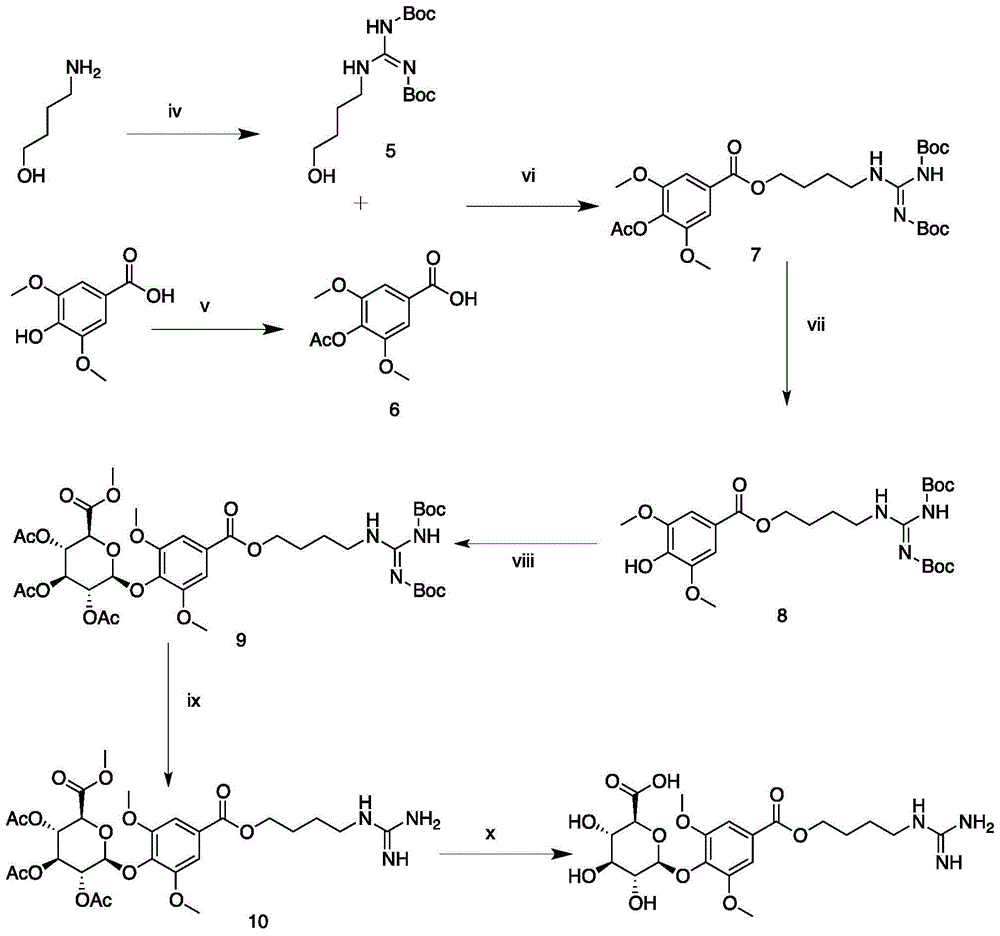
The invention relates to the technical field of organic chemistry, in particular to a synthetic method for leonurine. Gamma-butyrolactone is used as a starting material to be subjected to ammonolysis to obtain gamma-hydroxybutyric acid amide; the gamma-hydroxybutyric acid amide and acetyl syringic acid are subjected to a condensation reaction; a dehydration reaction and a reduction reaction are carried out to obtain leonurus amine; and the leonurus amine and S-methyl isothiourea sulfate are subjected to a reaction to obtain the leonurine. The target product leonurine is synthetized from the cheap industrial raw materials of the gamma-butyrolactone and the syringic acid used as the starting materials through reactions of ammonolysis, esterification, dehydration, reduction and the like. The reaction conditions are mild and easy to control; the yield is up to 65%; the product purity is 98% or above; and the synthetic method for leonutine provides the production with an excellent synthetic route and is suitable for large-scale production.
Owner:绵阳市润土农业科技开发有限公司
Chinese medicine agent for treating after medicament abortion uterus bleeding
InactiveCN101199655AReduce bleedingNo painSexual disorderBlood disorderComplete abortionHerbal preparations
The invention discloses a Chinese herbal preparation for treating uterine bleeding after drug abortion, which is made from following raw materials in weight proportion: 2 to 6 parts of astragalus root, 1.5 to 4 parts of agrimony, 0.9 to 2 parts of Chinese angelica, 2 to 4 parts of leonurine, 0.3 to 0.9 part of powdered notoginseng root, 1.5 to 3 parts of portulaca, 1 to 3 parts of redroot gromwell root, 0.8 to 2 parts of aspidium, 0.6 to 2 parts of bitter orange and 0.6 to 2 parts of dried rehmannia root. The invention integrates the functions of Qi supporting and blood enriching, muscle regeneration and hemostasis and heat clearing and blood cooling and cures uterine bleeding after drug abortion with safety, reliability and without pain and can obviously increase complete abortion rate and reduce bleeding quantity of the uterine to shorten bleeding time and avoid twice curettage.
Owner:薛晓彤
Method for synthesizing leonurine
ActiveCN105481724AHigh yieldAtom economy is highOrganic compound preparationOximes preparationS-methylisothioureaLeonurine
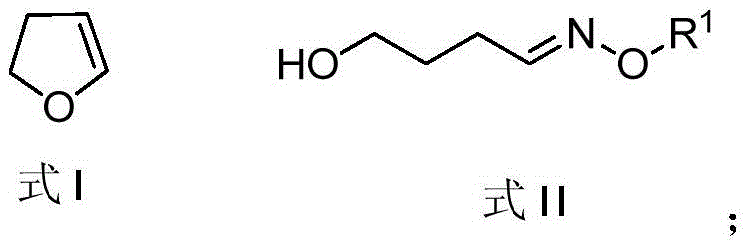
The invention discloses a method for synthesizing leonurine. The method is characterized in that raw material 2,3-dihydrofuran undergoes the alkoxy oxime synthesis reaction, obtained product 4-hydroxyl butyl-O-methyl oxime and carbalkoxy syringic acid undergo the esterification and oxime reduction reaction to obtain herba leonuri amine, and the herba leonuri amine reacts with S-methylisothiourea to obtain leonurine. The reaction process atom economy is good, the excessive use of a reagent is avoided, the environmental pollution is reduced, and the method is high in yield and easy to operate.
Owner:安徽省科学技术研究院
Leonurine derivative and application thereof in preparing medicine for preventing or treating ischemic cerebrovascular diseases
ActiveCN112552211AReduce the size of cerebral infarctionNovel structureOrganic active ingredientsNervous disorderAmino acid substitutionPharmacometrics
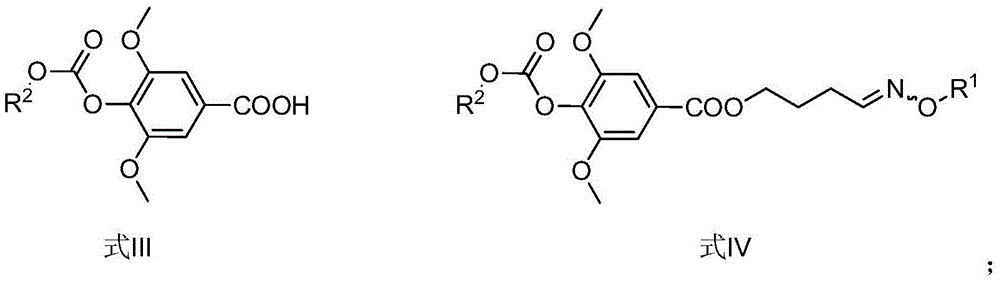
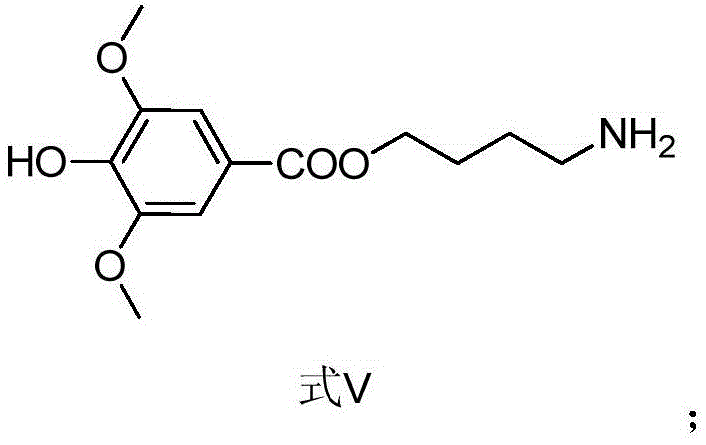
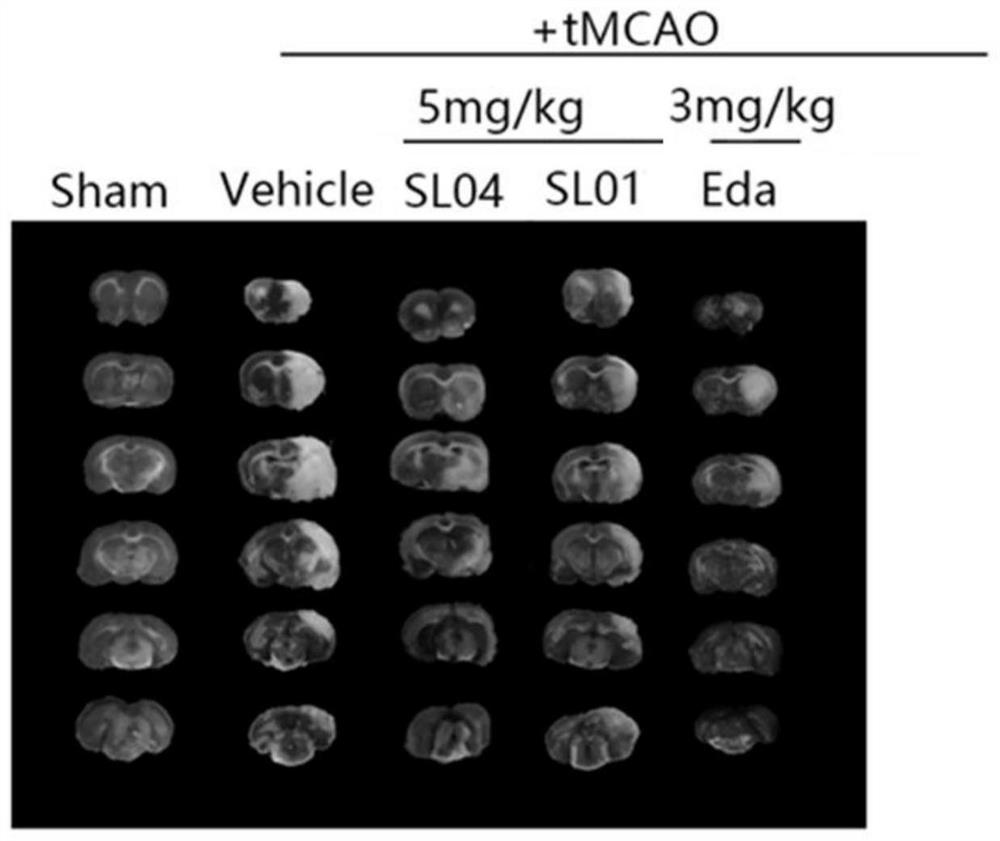
The invention provides a leonurine derivative and application of the leonurine derivative in preparation of a medicine for preventing or treating ischemic cerebrovascular diseases. The leonurine derivative has a structure as shown in a general formula (I), wherein X is selected from O or NH; Y is selected from any one of natural amino acid, substituted amino acid or amino alcohol; Z is selected from H, proline and any substituted proline. Pharmacological experiments prove that the leonurine derivative provided by the invention has the effects of neuroprotection, cerebral infarction area reduction and animal neurobehavioral scoring, and is good in safety, so that the leonurine derivative has important significance for developing novel medicines for preventing or treating ischemic cerebrovascular diseases.
Owner:青岛海合生物科技有限公司
Application of leonurine in preparing euglycemic agent
The invention belongs to the field of modern traditional Chinese medicine production, and relates to Chinese herbal medicine herba leonuri extract leonurine and application of the leonurine in preparing drugs, in particular to application of the leonurine in preparing a euglycemic agent and preventing the obesity. Through the pharmacological study and insulin sensibilization experiments, the results show that the leonurine has the effect of improving the sensibility of insulin in the body, and the leonurine can enhance the reaction of the body to the insulin, enhance the physiological effect of the insulin and increasing the sensibility of an insulin receptor; the experimental results show that under the condition that the leonurine is far lower than the toxic dose, the effect of reducingthe body weight is shown. The leonurine can be used for preparing the euglycemic agent and the drugs for preventing the obesity and is especially suitable for etiological treatment of the insulin resistance syndrome and the secondary disease symptoms caused by reduction of insulin resistance or insulin sensitivity, such as the central obesity, the simple obesity and the obesity with any other disease states.
Owner:ZHUHAI HENGQIN NEW DISTRICT ZHONGZHU ZHENGTAI MEDICAL MANAGEMENT CO LTD
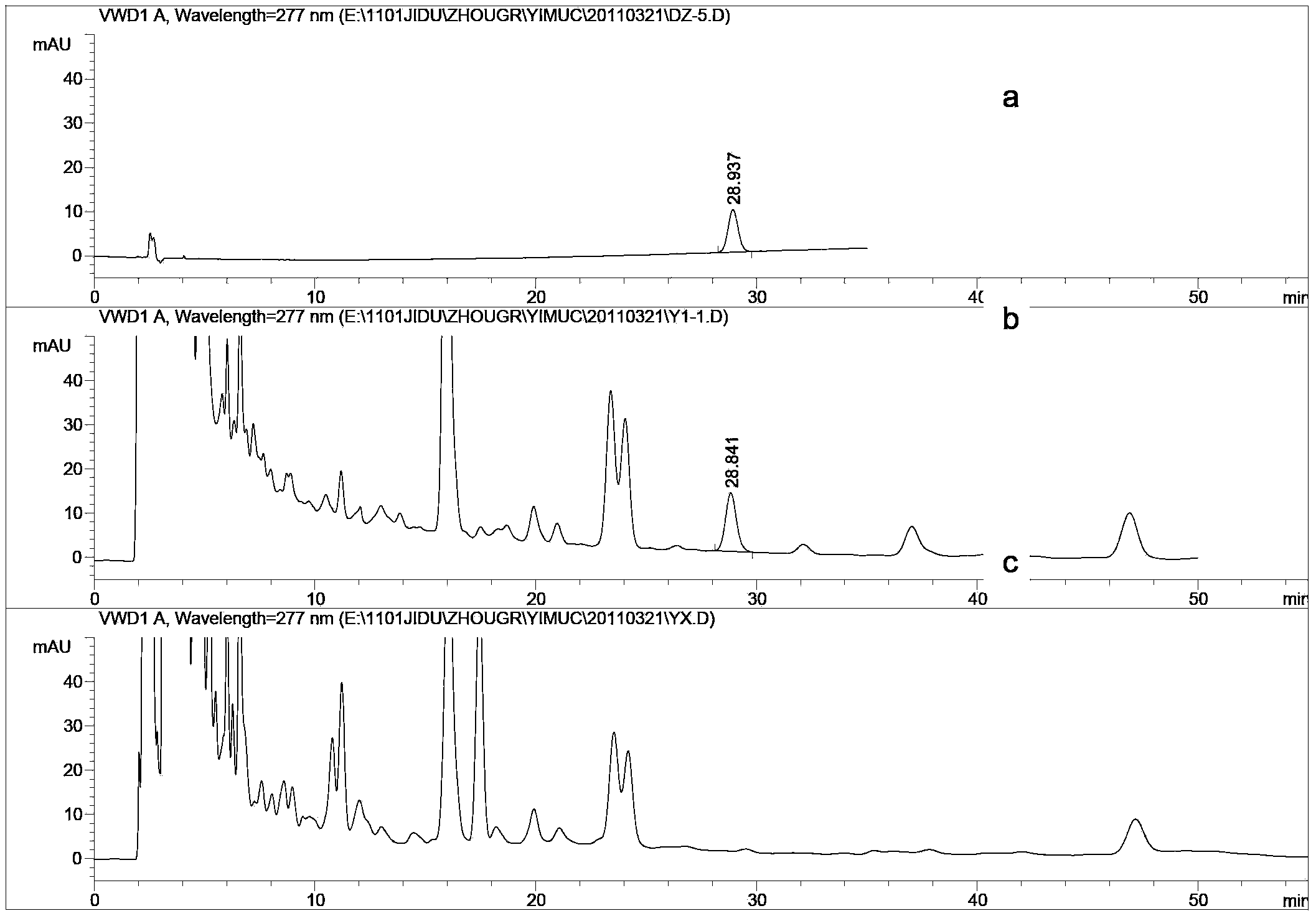
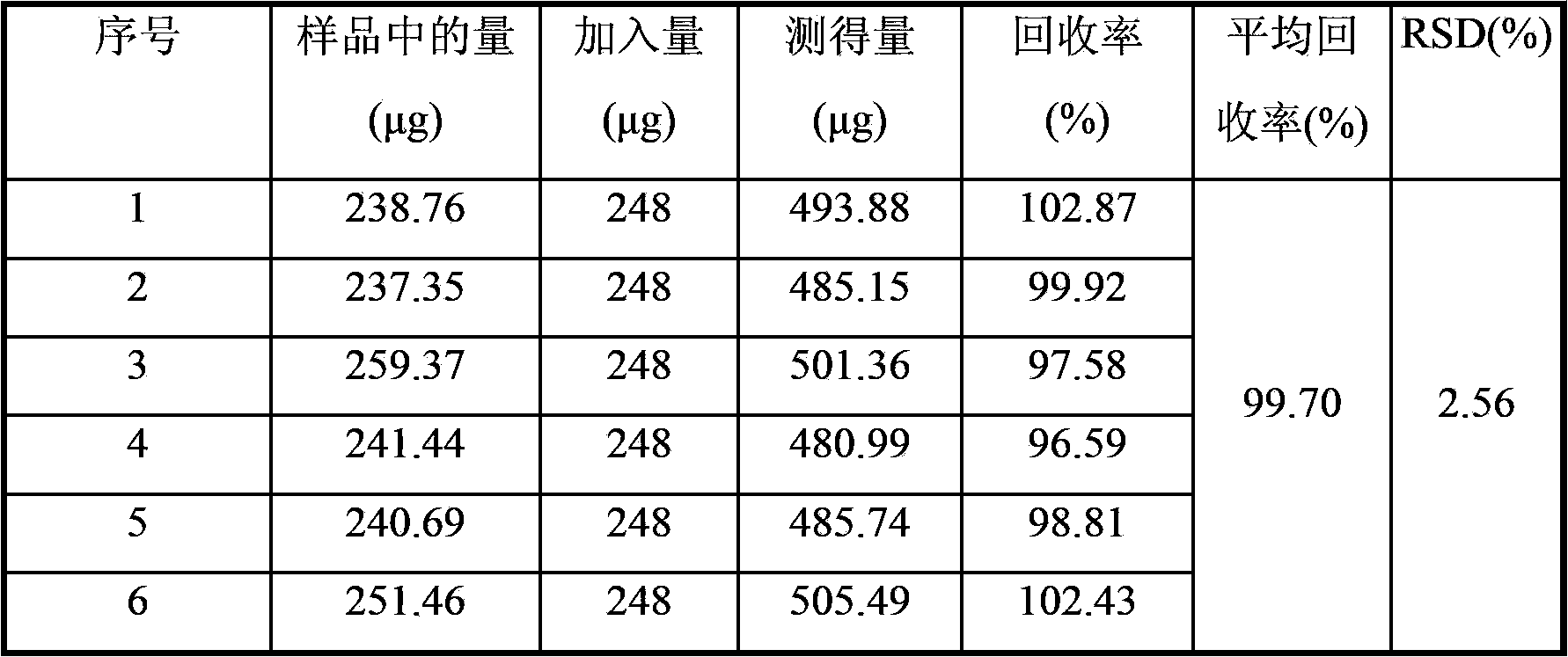
Method for detecting leonurine hydrochloride content in nephritis elimination tablet
ActiveCN103575816AEasy to separateGood reproducibilityComponent separationSodium 1-octanesulfonatePhosphoric acid
The invention discloses a method for detecting the leonurine hydrochloride content in a nephritis elimination tablet. The detection method adopts a reversed phase ion pair chromatography method to measure the leonurine hydrochloride content in a nephritis elimination tablet, wherein the chromatographic condition adopts a C18 column, the mobile phase is acetonitrile-0.1% phosphoric acid solution of 0.2-0.6% sodium 1-octanesulfonate, and the detection wavelength is 260 to 290 nm. The detection method has the advantages of convenient operation, high sensitivity, and good repeatability, and provides a reference for establishment of quality standards for the nephritis elimination tablet.
Owner:天津天士力圣特制药有限公司
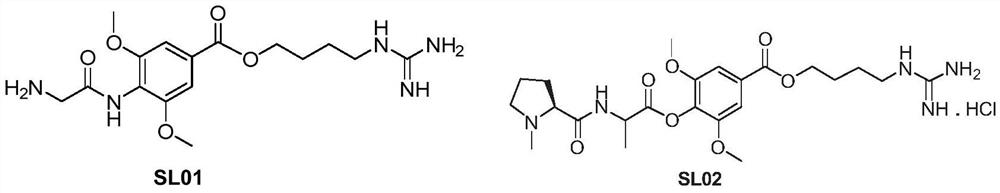
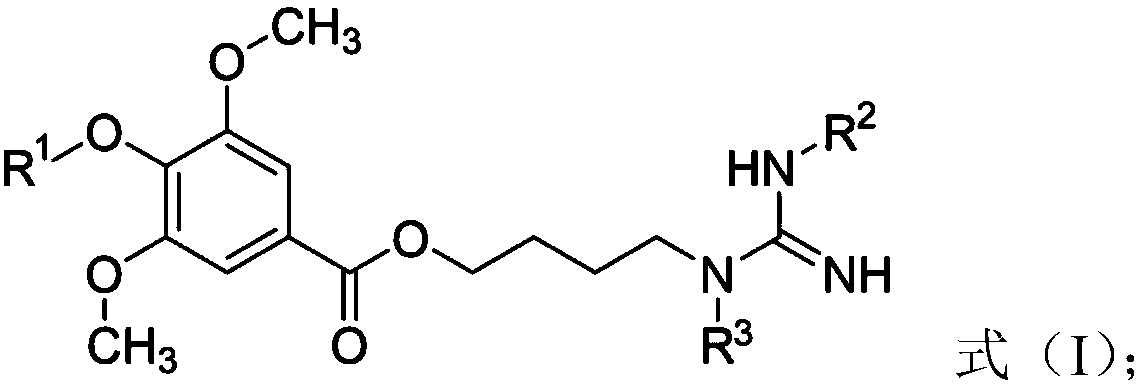
Leonurine derivative as well as preparation method and application thereof
ActiveCN108840808AImprove oral bioavailabilityReduce stimulationOrganic chemistryOrganic compound preparationHydrogenLeonurine
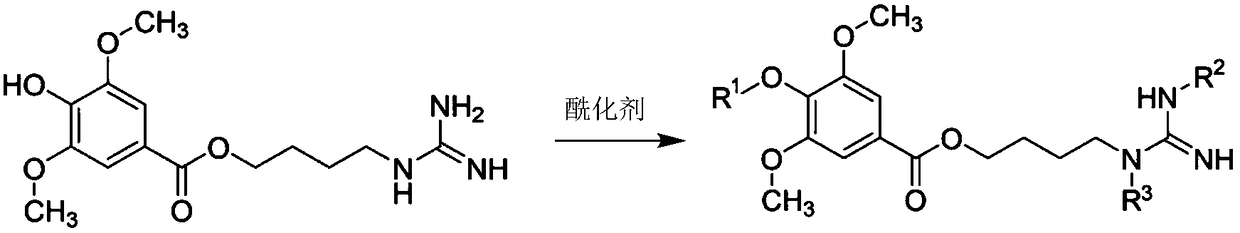
The invention discloses a leonurine derivative as well as a preparation method and application thereof. In a compound and pharmaceutically acceptable salt or prodrug thereof as shown in a formula (I),R1, R2 and R3 are the same or different and are independently selected from hydrogen, C1-C10 linear chain or branched alkyl carbonyl or C1-C10 linear chain or branched alkoxyl carbonyl. Relative to the leonurine in the prior art, the leonurine derivative as shown in the formula (I) has the following advantages: the bioavailability of the leonurine derivative in the animal body is greatly improved; furthermore, the leonurine derivative in blood is hydrolyzed into leonurine rapidly, and the condition of low exposed quantity caused by the fact that the leonurine is absorbed insufficiently in theliving body is improved. (The formula is as shown in the description).
Owner:北京合力众盈医药科技有限责任公司
Medicine for resisting pressure overload myocardial hypertrophy and ventricular remodeling and application
InactiveCN112773784AInhibit hypertrophyImproved cardiac diastolic dysfunctionCompounds screening/testingOrganic active ingredientsStainingBlood flow
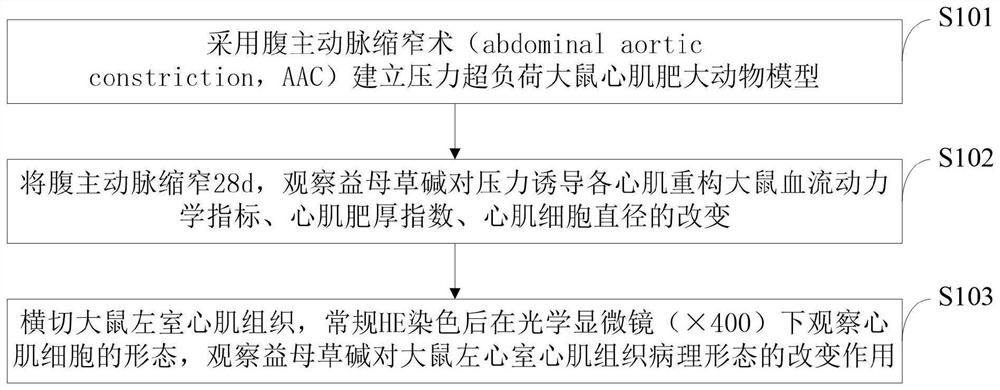
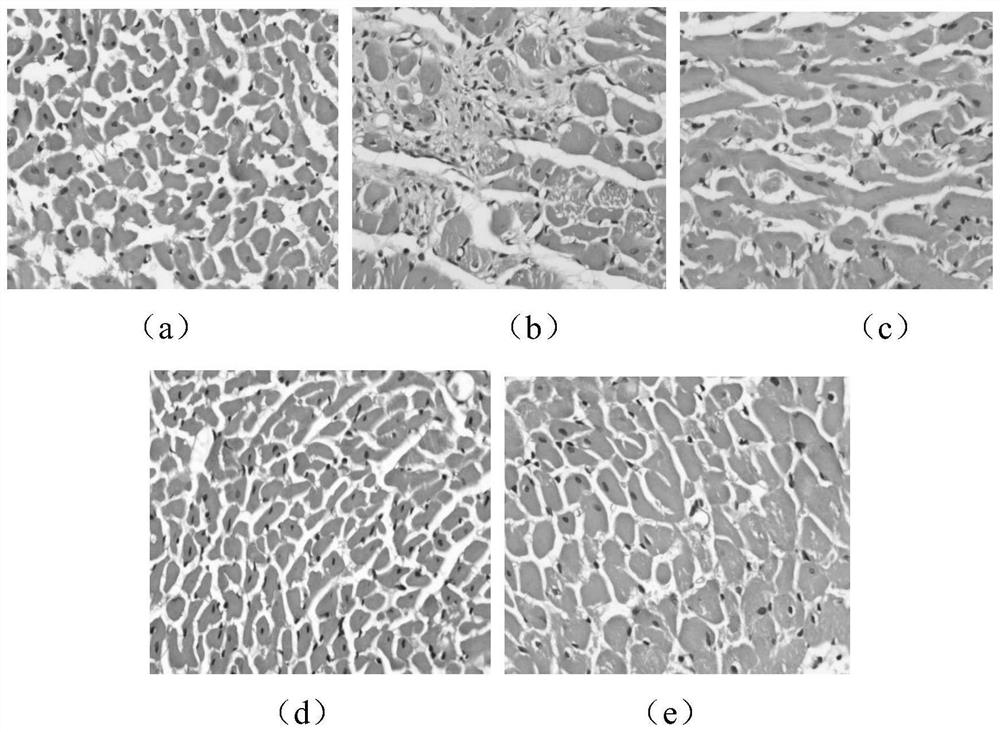
The invention belongs to the technical field of biological pharmacy, and discloses a medicine for resisting pressure overload myocardial hypertrophy and ventricular remodeling and application. The application comprises the following steps of: establishing a pressure overload rat myocardial hypertrophy and ventricular remodeling animal model by adopting abdominal aorta constriction; constricting the abdominal aorta for 28d, and sequentially detecting the change of a hemodynamic index and the diameter of a myocardial cell; and transecting the left ventricular myocardial tissue of a rat, observing the form of the myocardial cell under an optical microscope after conventional HE staining, and observing the change effect of leonurine on the pathological form of the left ventricular myocardial tissue of the rat. The invention proves that high and low dosage groups of leonurine have a certain improvement effect on cardiac diastolic and systolic dysfunction of a myocardial hypertrophy rat induced after abdominal aorta constriction; the high and low dosage groups of leonurine can reduce the left ventricular hypertrophy index of the rat after AAC operation; and the high and low dosage groups of leonurine and a positive group can inhibit myocardial cell hypertrophy of the rat after AAC operation and inhibit ventricular remodeling.
Owner:贵州中医药大学
Beta crystal form of leonurine hydrochloride as well as preparation method and application of beta crystal form
InactiveCN108276308AImprove stabilityOrganic active ingredientsOrganic compound preparationHigh humidityX-ray
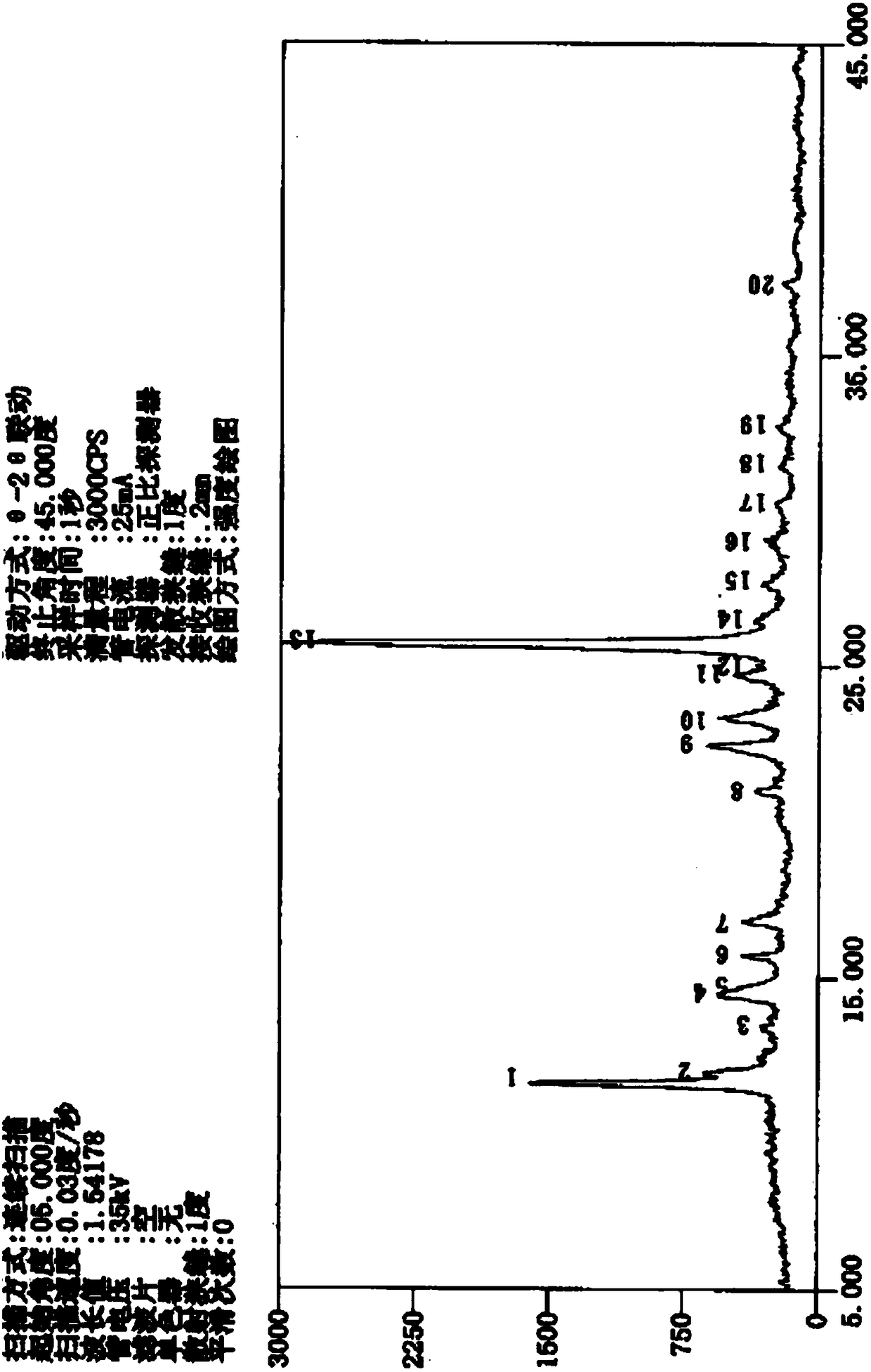
The invention discloses a beta crystal form of leonurine hydrochloride as well as a preparation method and application of the beta crystal form. Stronger diffraction peaks displayed in a powder X-raydiffraction pattern expressed by diffraction angles 2theta are 11.630 (54 percent), 11.990 (21 percent), 14.420 (18 percent), 22.430 (21 percent), 23.330 (19 percent), 24.710 (16 percent) and 25.670 (100 percent). The beta crystal form of the leonurine hydrochloride, which is prepared with the preparation method disclosed by the invention, has the characteristic of high stability, can keep stableunder conditions of high illumination, high temperature, high humidity and long-time storage, and can be used for preparing a drug composition.
Owner:旌德新星生物科技有限公司
Extracting method of green tea extracting solution and tea beverage for treating hyperlipemia
InactiveCN106212801ASimple structureEasy to operateOrganic active ingredientsMetabolism disorderAlcohol contentFiltration
The invention provides an extracting method of a green tea extracting solution. The extracting method comprises the steps that fresh green tea is taken, cleaned and triturated, water 10 times by weight of the tea is added, soaking is performed for 0.5 hour, decoction is performed for 1 hour, filtering is performed, water 8 times by weight of medicinal materials is added to decoction dregs, decoction is performed for 1 hour, medicinal liquid is filtered out, two times of decoction liquid are mixed, concentration is performed to obtain 60 DEG C liquid with the relative density of 1.05, ethanol is added to make the volume percentage of alcohol content up to 75%, stirring, standing and filtration are performed, filtrate is concentrated to obtain 65 DEG C liquid with the relative density of 1.25, the ethanol is recovered to obtain concentrate, the green tea extracting solution is obtained through microwave extraction, the tea polyphenol content is high, and the green tea extracting solution is compatible with leonurine and forsythin to make a tea beverage.
Owner:NANJING ZHENGKUAN MEDICAL TECH
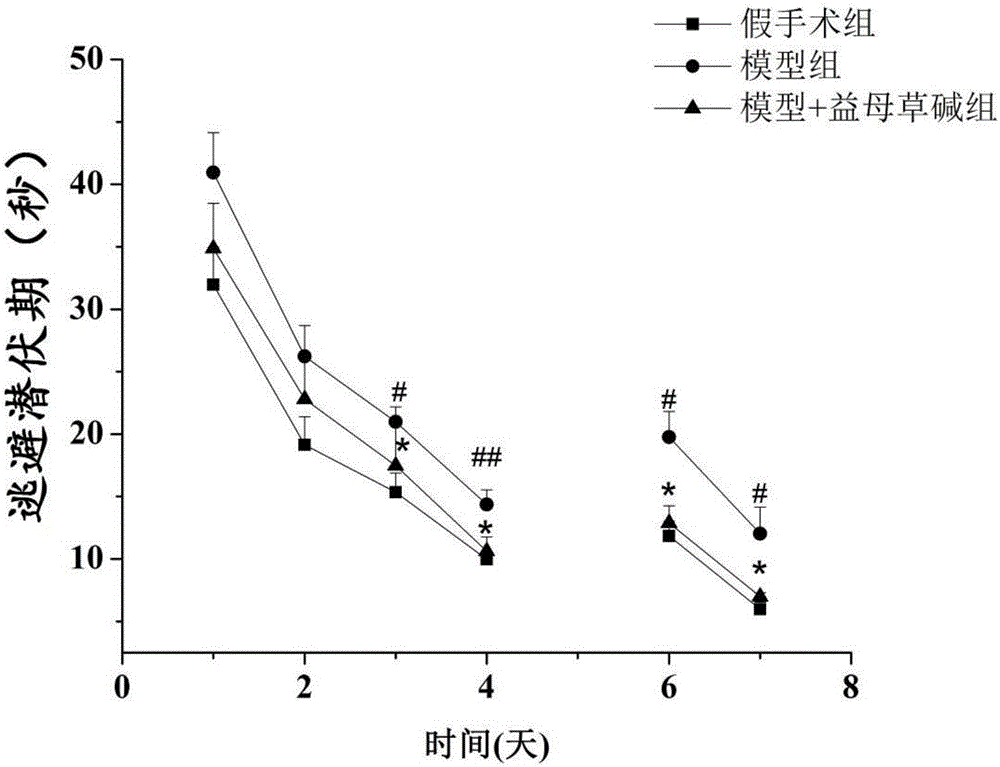
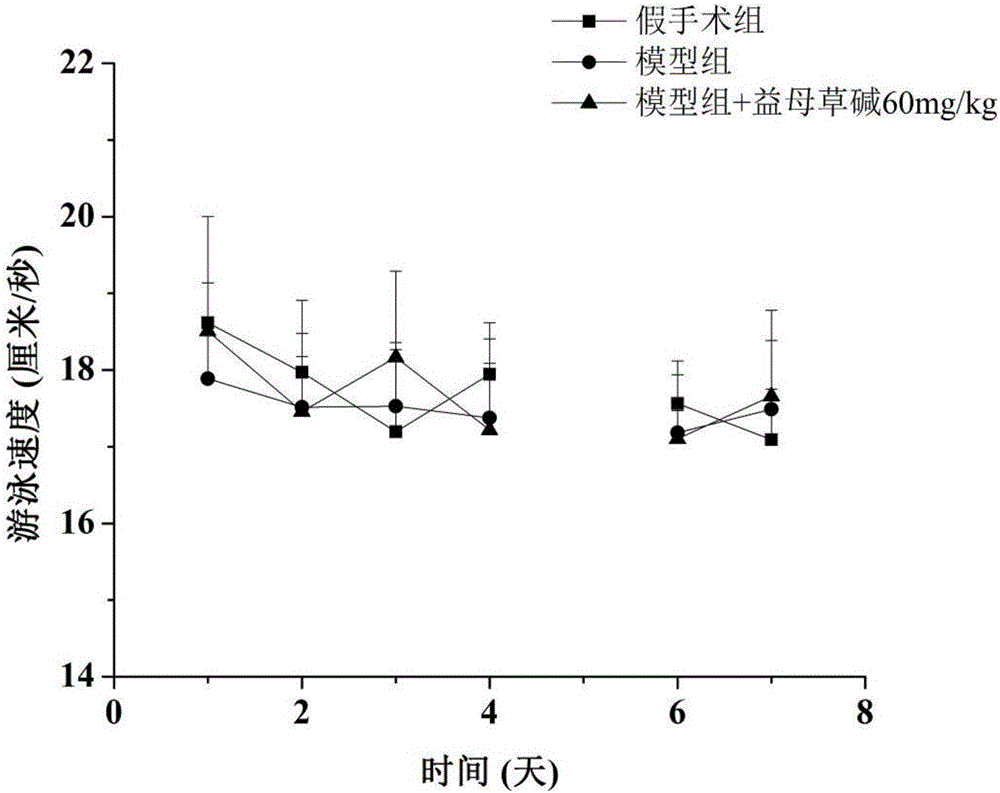
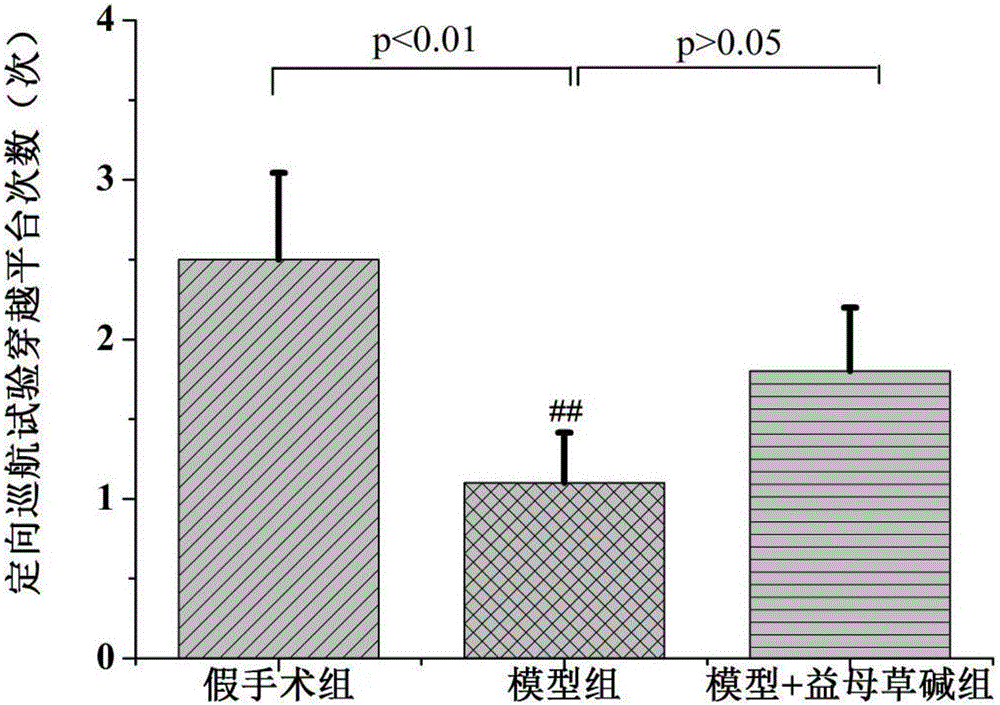
Application of leonurine to preparation of medicine for treating vascular dementia
InactiveCN105997975AShortened escape latencyIncrease Target Quadrant PercentageOrganic active ingredientsNervous disorderPharmacyInjury brain
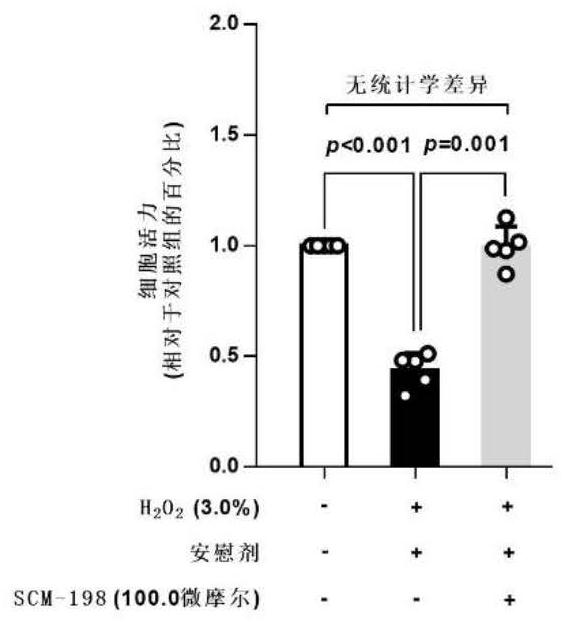
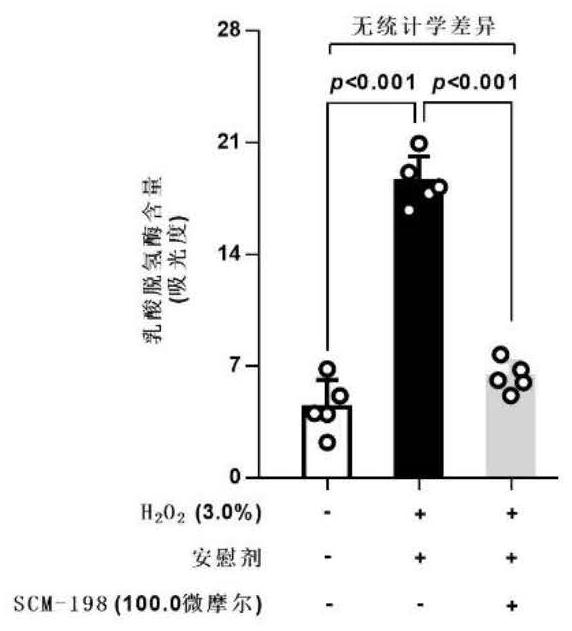
The invention belongs to the field of pharmacy, relates to application of leonurine to preparation of a medicine for treating dementia and in particular relates to application of the leonurine to preparation of a medicine for treating vascular dementia. A rat VD model is successfully copied and an experiment result shows that the leonurine can remarkably improve cognitive impairment caused by the VD and alleviate impairment of long-term postsynaptic inhibition; the content of hydrogen peroxide (H2O2) and glutamate is reduced, the specific value of NR2A / 2B is improved and the expression of LC3II / LC3I and Beclin-1 is regulated down, so that autophagic flux is inhibited. The leonurine can be used as a treatment medicine for treating the dementia, especially brain injury diseases caused by the VD.
Owner:NANKAI UNIV +1
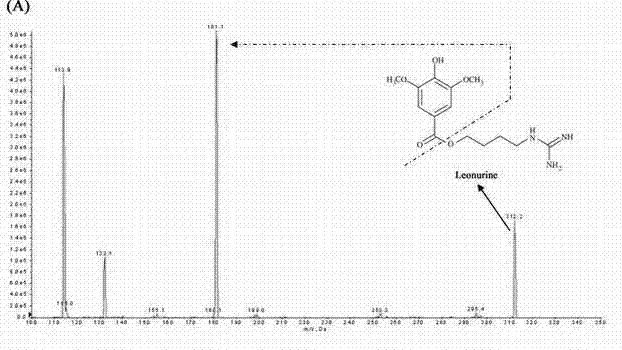
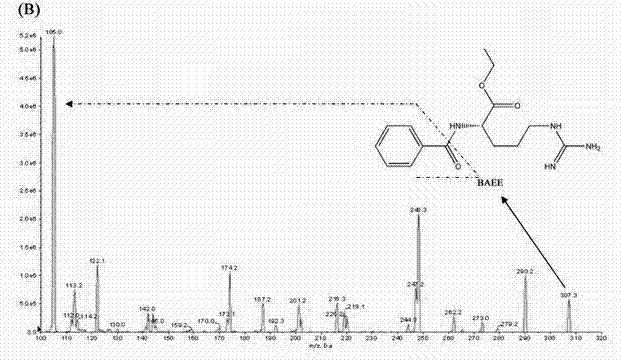
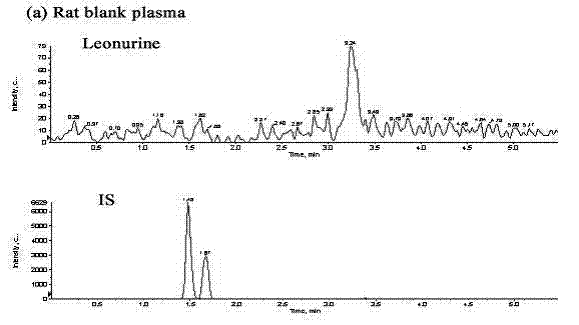
Method for quantitatively detecting content of leonurine in blood plasma
ActiveCN102680620AReduce interferenceHigh sensitivityComponent separationArginine ethylesterArginine
The invention belongs to the technical field of medicine detection and relates to a method for quantitatively detecting the content of leonurine in blood plasma. The method is characterized by adopting the high performance liquid chromatography-mass spectrometry technology, using benzoyl arginine ethyl ester as an internal standard of a detected substance, using a method of precipitating the proteins with perchloric acid to pretreat the blood plasma sample and calculating the blood concentration of leonurine in the blood plasma by using the peak area according to an internal standard method, wherein liquid chromatography adopts Agilent ZorbaxSB-C18 column as the stationary phase, the acetonitrile / ammonium acetate buffer solution mixed solution is used as the mobile phase and gradient elution is carried out; mass spectrometry adopts an electrospray ionization source; leonurine is quantified by adopting multiple reaction monitoring; and the lowest limit of quantification of leonurine in the method reaches 4ng / mL. The method has the following beneficial effects: the method has strong sample detection specificity and high sensitivity and is simple and convenient to operate; all the textual research indexes of methodology can meet relevant technical requirements of Chinese pharmacopoeia; and the method can be used for pharmacokinetics research after oral administration of leonurine.
Owner:ZHUHAI HENGQIN NEW DISTRICT ZHONGZHU ZHENGTAI MEDICAL MANAGEMENT CO LTD
Method for producing cell activator beauty cream by taking plant extracts as main functional ingredients
InactiveCN107137317AIncrease the speed of survival metabolismBlock generationCosmetic preparationsToilet preparationsHuman bodyAdditive ingredient
The invention discloses a method for producing cell activator beauty cream by taking plant extracts as main functional ingredients. The production process of the cell activator beauty cream is as follows: 1, preparation of chinaroot greenbrier cream, 2, extraction of motherwort and leonurine, 3, extraction of paeoniflorin and paeonin, 4, production of poria cocos flavor powder, and 5, preparation of matrix buteprate cream. Cell activator is artificially extracted from plants and is made into a cream shape so as to be applied onto the surface of a human body, so that tyrosine enzymatic activity is inhibited, melanogenesis is blocked, main active materials such as flavone, saponin and the like are activated, cell activating reaction is triggered, cell survival and metabolism speed is improved, collagen is promoted to generate, such effects as whitening, freckle removing, skin nourishing, ageing delaying and free radical scavenging are generated, as the beauty cream is focused on nursing as a whole, emphasis is put on inner nursing firstly, and then a cosmetic effect shows outside. Therefore, the cosmetic effect is durable and stable, and the beauty cream has a pure natural attribute, and is characterized by being non-toxic, harmless, noncarcinogenic and the like.
Owner:李青
Leonurine metabolite and preparation method thereof
InactiveCN106146573AEasy to operateSimple reaction conditionsSugar derivativesSugar derivatives preparationChemical structureMetabolite
The invention belongs to the field of medicine synthesis, and particularly relates to a leonurine main metabolite and a preparation method thereof. The main metabolite which has a structure shown in the formula (I) (please see the formula in the description) and combines leonurine with glucuronic acid is synthesized by taking glucuronic acid lactone, 4-aminobutanol and syringic acid as starting raw materials through multi steps of reactions. The prepared leonurine main metabolite compound which has the novel chemical structure and combines leonurine with glucuronic acid has a good pharmacological activity prospect and can be used for further pharmacological activity evaluation. The method has the advantages that the synthetic route is simple, control is easy, the raw materials are cheap and easy to obtain, and the product stability is good.
Owner:FUDAN UNIV
Novel application of leonurine
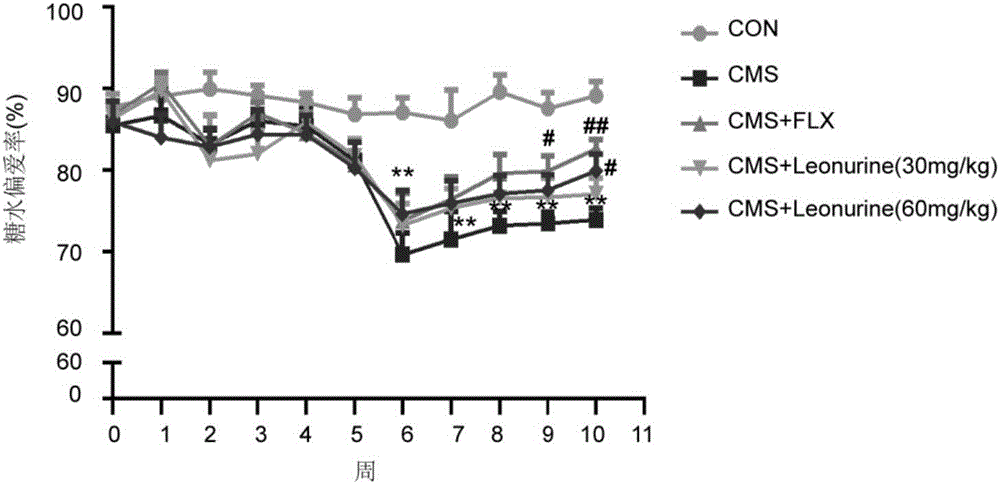
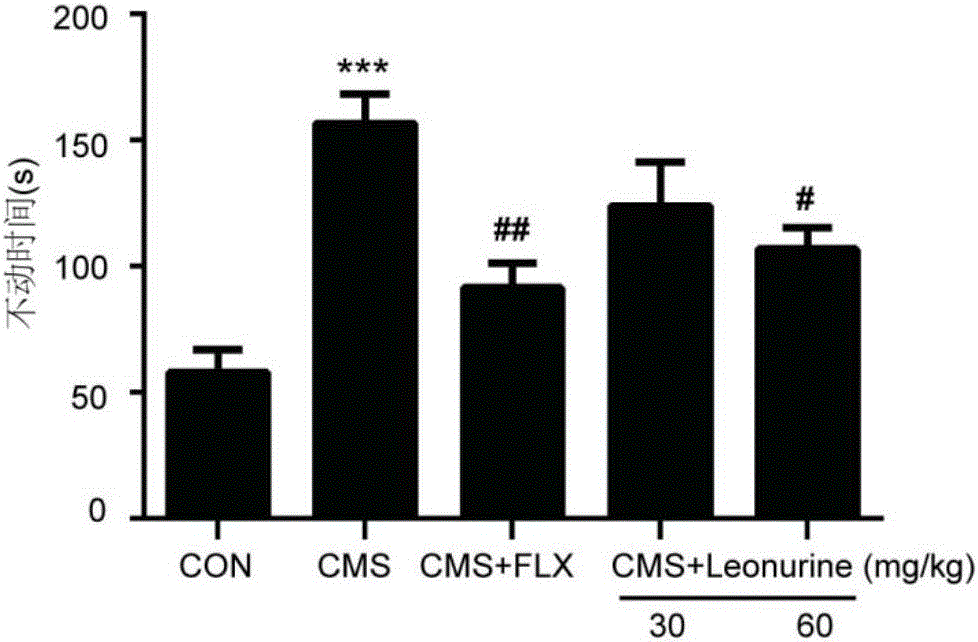
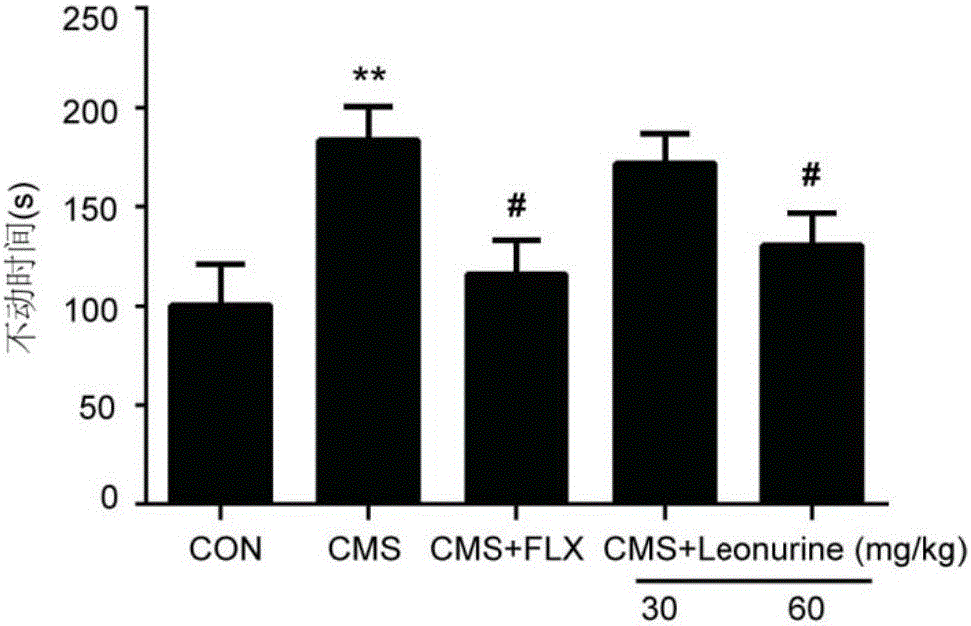
The invention belongs to the field of traditional Chinese medicine pharmacy and relates to application of leonurine in the preparation of drugs for preventing or treating depression. After depression-like symptoms in a mouse are induced through a chronic mild stress model, leonurine is given via gavage; the results indicate that leonurine can significantly improve depression-like behaviors of the CMS mouse, increase the mass transfer contents of 5-HT, NE and DA monoamines, improve hippocampal neuronal injury, increase the number of astroglia cells in sea horse, inhibit cerebral neuritis, and increase the levels of cerebral nerve growth factors BDNF and GDNF. It can be seen from the invention that leonurine resists depression in multiple terms and is suitable for the preparation of drugs for preventing or treating depression.
Owner:NANJING MEDICAL UNIV
Virus immunized inducer and application thereof
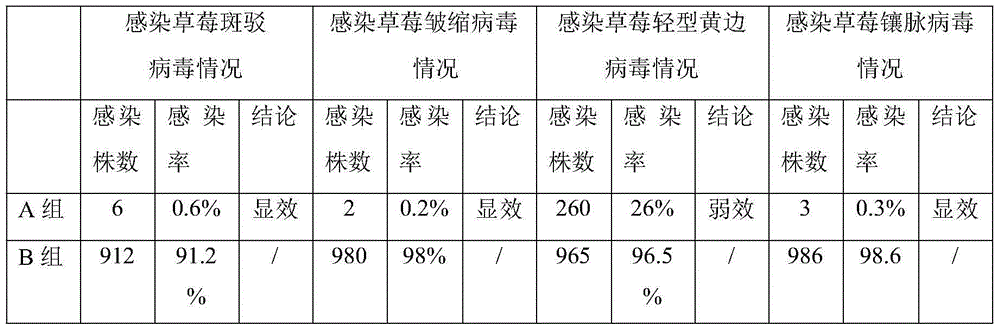
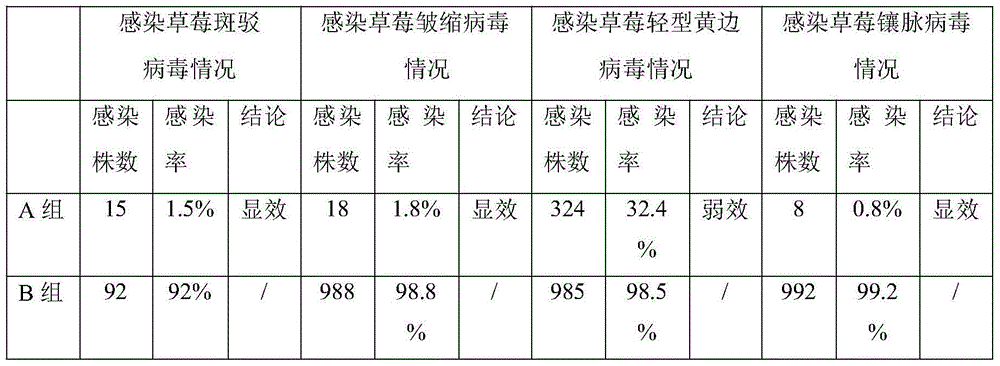
The invention discloses a virus immunized inducer and application thereof, and belongs to the technical field of plant virus immunized induction. The inducer is prepared by the following steps: uniformly mixing the following components in percentage by weight: 32 percent of astragalus polysaccharide, 26 percent of chlorogenic acid, 26 percent of total tanshinones and 16 percent of leonurine; and adding 8.5 percent of the mixture in a root medium for strawberry virus-free seedling tissue culture. The immunized inducer has the advantages of effectively improving the resistance of strawberry virus-free seedlings on viruses, has excellent immunity on strawberry mottle virus, crinkle virus and vein banding virus in the natural planting process, and has minor immunity on strawberry mild yellow edge virus.
Owner:武汉勤劳农夫生态农业有限公司
Leonurine hydrochloride tablet and preparation method thereof
ActiveCN112933056AAvoid residueThe raw materials of the preparation process are cheap and easy to obtainOrganic active ingredientsPill deliveryAdhesiveOrganosolv
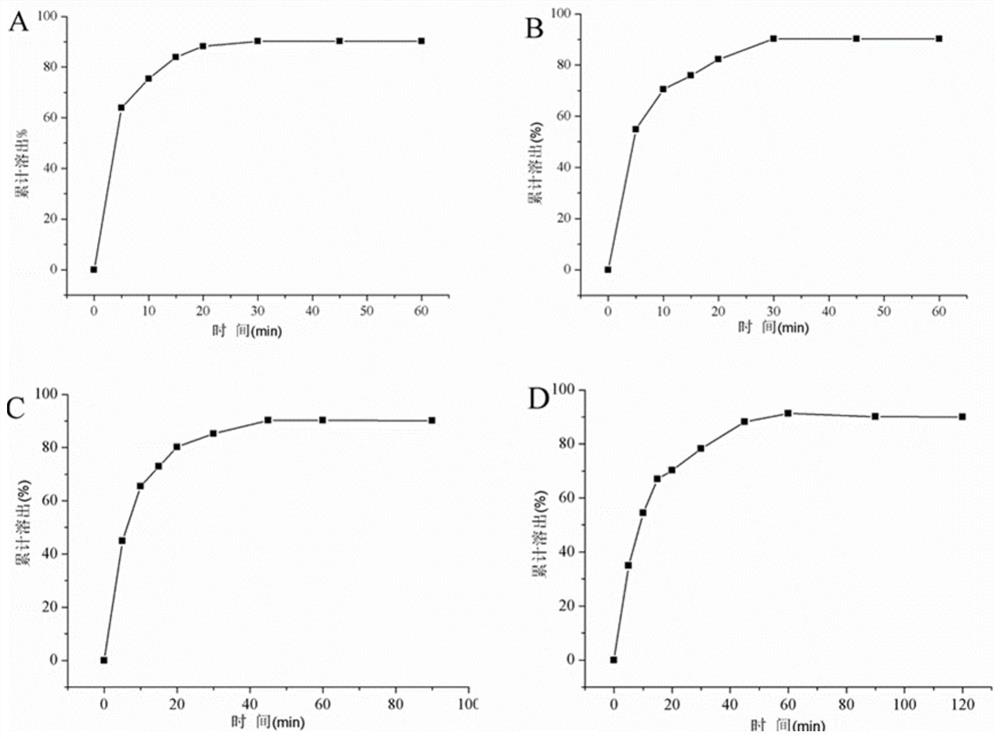
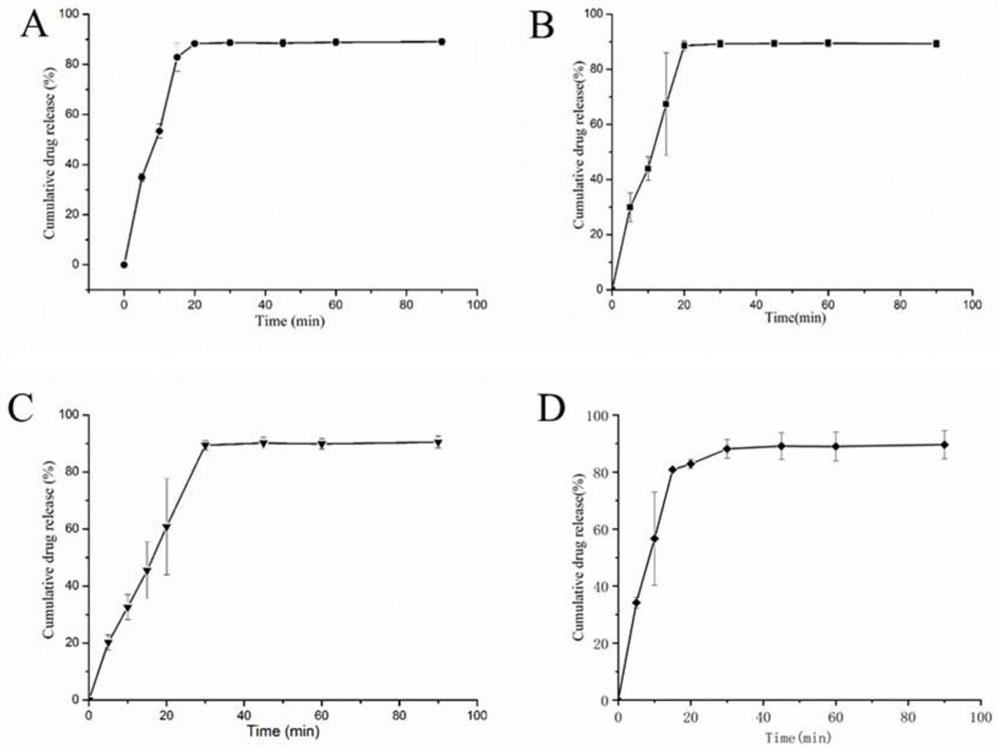
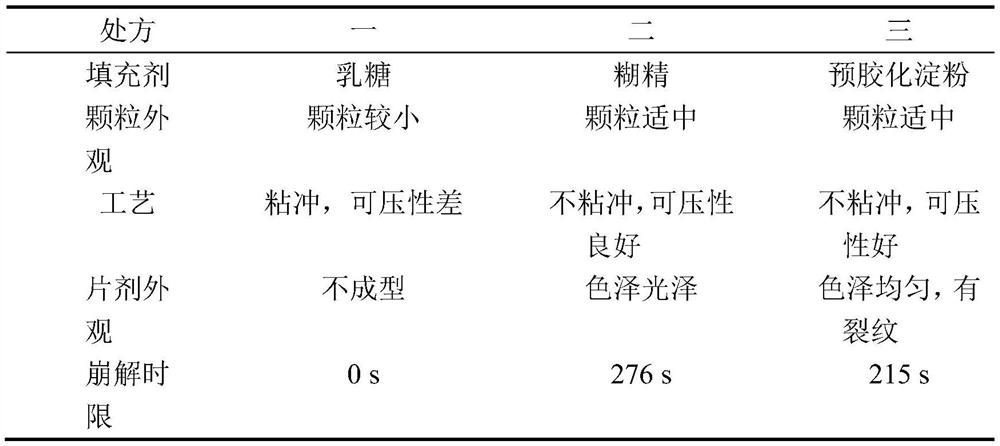
The invention discloses a leonurine hydrochloride tablet and a preparation method thereof, and belongs to the technical field of medicines. The tablet prepared by the invention mainly comprises the following components in parts by weight: 500 mg of leonurine hydrochloride, 8750 mg of a filler, 500 mg of a disintegrating agent, 200 mg of an adhesive and 50 mg of a lubricant. In the preparation process, the leonurine hydrochloride, the filling agent and the disintegrating agent are firstly mixed, then the adhesive aqueous solution is added to prepare a soft material, then granulation is performed, and finally tabletting and coating are performed on the soft material, the lubricant and the adhesive dry powder. The preparation process comprises the steps of mixing of raw materials and auxiliary materials, wet granulation, drying, size stabilization, total mixing and tabletting. The leonurine hydrochloride raw material medicine is prepared into tablets for the first time; organic solvents such as ethanol are not used in the preparation method, so that organic solvent residues are avoided; the dissolution rates of the leonurine hydrochloride tablets prepared by the invention in water dissolution media with the pH values of 1.2, 4.5 and 6.8 respectively reach about 85% within 30 minutes. The preparation process is cheap and easily available in raw materials, stable in quality and suitable for large-scale industrial production.
Owner:NANJING UNIV OF TECH
Application of leonurine to prevention of acute liver injury and promotion of repair and regeneration of liver tissue
The invention discloses an application of leonurine to preparation of medicines for preventing acute liver injury and medicines for promoting repair and regeneration of liver tissue. The leonurine hasprotection effects on the liver tissue, and can prevent the acute liver injury and can promote repair and regeneration of the liver tissue.
Owner:王瑞
Application of leonurine in preparation of medicine for improving infertility pregnancy outcome
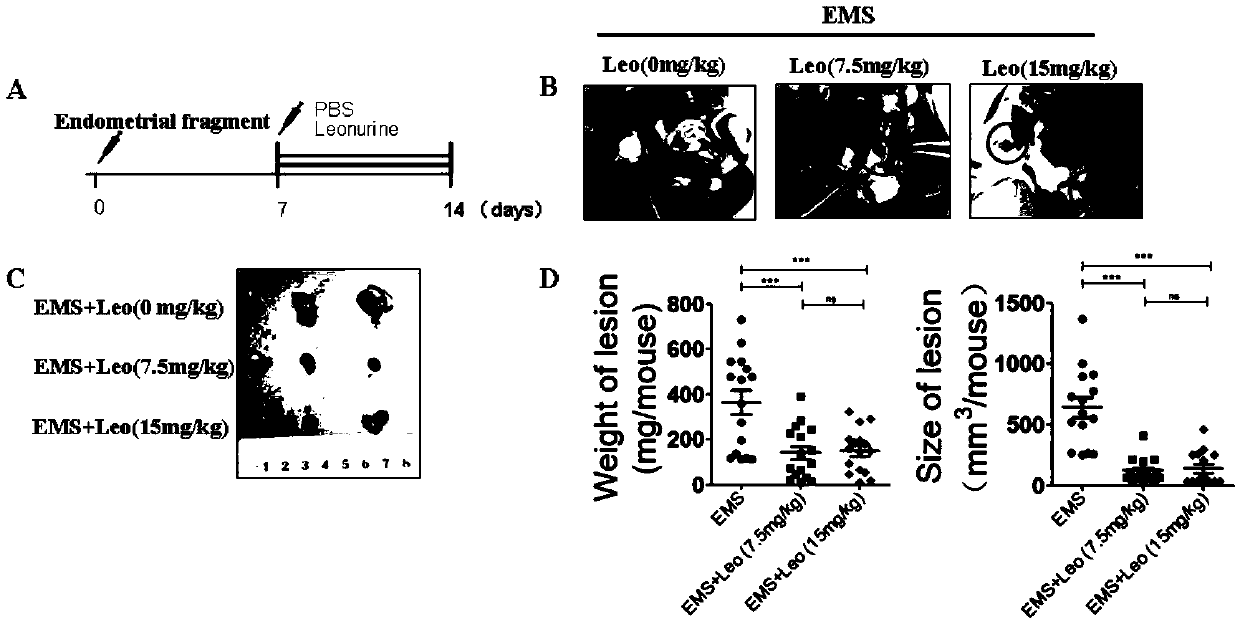
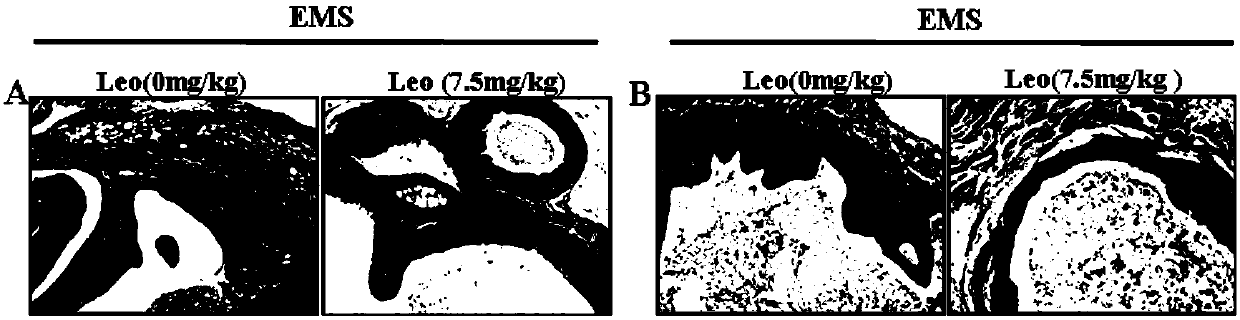
PendingCN111374967AImprove pregnancy rateWeaken invasive metastasisOrganic active ingredientsSexual disorderPhysiologyApoptosis
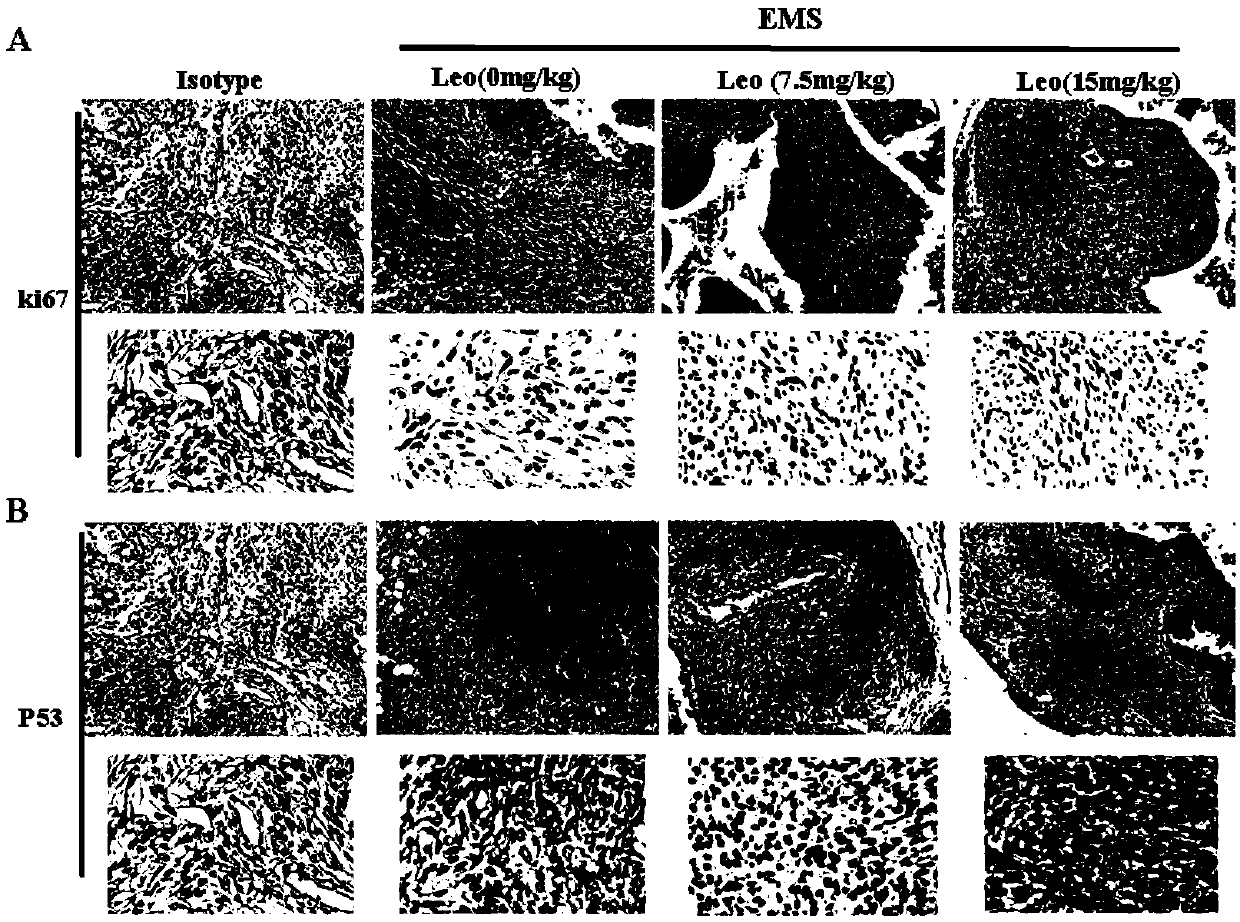
The invention belongs to the field of modern pharmacy of traditional Chinese medicines, and relates to application of a specific monomer leonurine in preparation of a medicine for improving infertility pregnancy outcome. The leonurine is injected into the abdominal cavity during an EMS (acute renal syndrome) modeling period, so that the volume and weight of an ectopic focus can be remarkably reduced, local fibrosis, invasion and transfer are inhibited, and the pregnancy rate of an EMS model mouse can be remarkably increased. The leonurine inhibits proliferation and invasion of endometrium stromal cells by inducing apoptosis of the endometrium stromal cells, adjusts expression balance of a local estrogen-progestin receptor of the ectopic focus, activates EMS model pelvic and abdominal immune cells, enhances phagocytic and killing functions of the cells, reverses poor ecdysis of the EMS model mouse, has similar effects on growth and invasion of ectopic focus ESC of a human EMS patient and expression of the estrogen-progestin receptor, does not affect growth and activity of the endometrium ESC of a normal child-bearing female, hormone receptor expression, ecdysis process and proliferation, apoptosis and cell activity of human early-pregnancy embryo trophoblast, and can be used for preparing an effective prevention and treatment medicine for EMS combined infertility patients.
Owner:THE OBSTETRICS & GYNECOLOGY HOSPITAL OF FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com