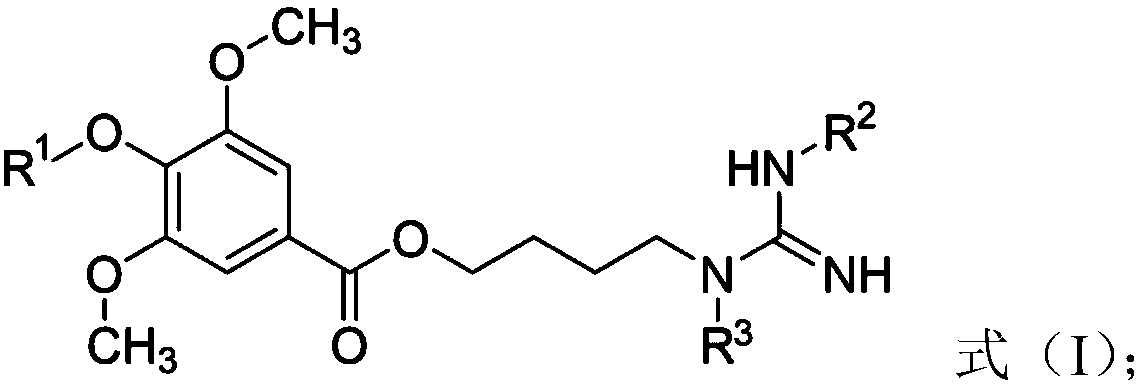
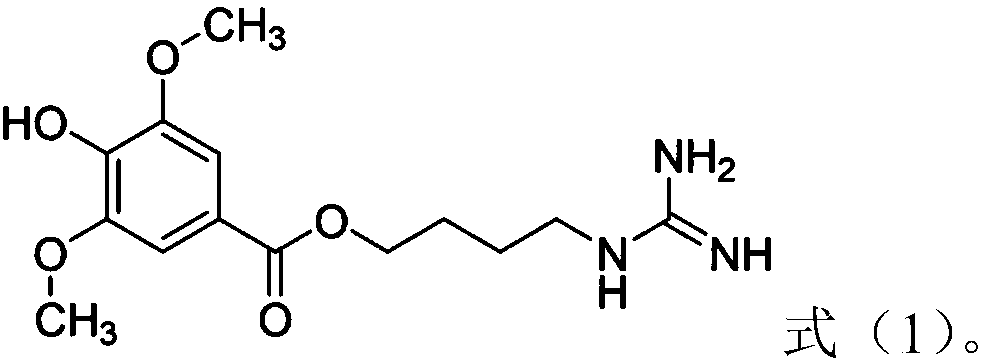
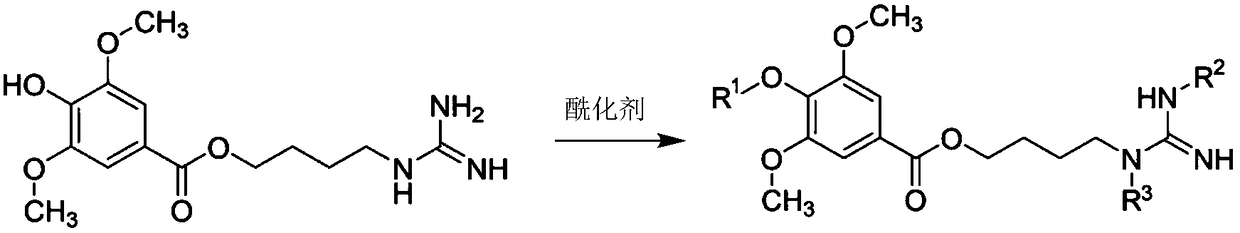
Leonurine derivative as well as preparation method and application thereof
A technology of leonurine and derivatives, which is applied in the field of drug synthesis, can solve problems such as low bioavailability of leonurine, and achieve the effects of improving oral bioavailability, cheap and easy-to-obtain raw materials, and easy control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Through the following chemical reaction equation, compound (II) is prepared:
[0041]
[0042] Add 100ml of tetrahydrofuran and 20ml of water into a 250ml three-necked flask, add 3.5g (0.01mol) of motherurine hydrochloride and 3.4g (0.04mol) of sodium bicarbonate, respectively, and stir at 20°C for ten minutes, then add 0.2g of N, N- For dimethyl-4-aminopyridine, 2.45g (0.02mol) of n-propyl chloroformate was dissolved in 10ml of tetrahydrofuran, and the resulting solution was slowly dripped into the reaction flask at 20°C, and continued stirring for 3 hours to After the reaction was completed, tetrahydrofuran was evaporated under reduced pressure, 70ml of saturated brine and 30ml of ethyl acetate were added for extraction, the aqueous layer was extracted twice with 20ml of ethyl acetate respectively, the organic layers were combined, washed twice with dilute citric acid solution, and the organic layer was separated. layer, added 3g of anhydrous sodium sulfate to dry,...
Embodiment 2
[0044] Through the following chemical reaction equation, compound (Ⅲ) is prepared:
[0045]
[0046] Add 200ml of tetrahydrofuran and 20ml of water into a 250ml three-necked flask, add 3.5g (0.01mol) of motherurine hydrochloride and 3.4g (0.04mol) of sodium bicarbonate, and stir at 25°C for ten minutes, then add 0.2g of N, N- Dimethyl-4-aminopyridine, take 2.74g (0.02mol) of isopropyl chloroformate and dissolve in 10ml of tetrahydrofuran, and slowly drop the resulting solution into the reaction flask at 25°C, and continue stirring for 3 hours to After the reaction was completed, tetrahydrofuran was evaporated under reduced pressure, 70ml of saturated brine and 30ml of ethyl acetate were added for extraction, the aqueous layer was extracted twice with 20ml of ethyl acetate respectively, the organic layers were combined, washed twice with dilute citric acid solution, and the organic layer was separated. layer, added 3g of anhydrous sodium sulfate to dry, filtered, and the fil...
Embodiment 3
[0048] Through the following chemical reaction equation, compound (Ⅳ) is prepared:
[0049]
[0050] Add 400ml of tetrahydrofuran and 30ml of water into a 250ml three-necked flask, add 3.5g (0.01mol) of motherurine hydrochloride and 5.1g (0.06mol) of sodium bicarbonate, and stir at 23°C for ten minutes, then add 0.3g of N, N- Dimethyl-4-aminopyridine, take 4.44g (0.03mol) of n-heptanoyl chloride and dissolve it in 20ml of tetrahydrofuran, and slowly drop the resulting solution into the reaction flask at 23°C, and continue stirring for 3.5 hours until the reaction is completed. After the end, the tetrahydrofuran was evaporated under reduced pressure, 80ml of saturated brine and 40ml of ethyl acetate were added for extraction, the aqueous layer was extracted twice with 30ml of ethyl acetate respectively, the organic layers were combined, washed twice with dilute citric acid solution, and the organic layer was separated , adding 6g of anhydrous sodium sulfate for drying, filte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com