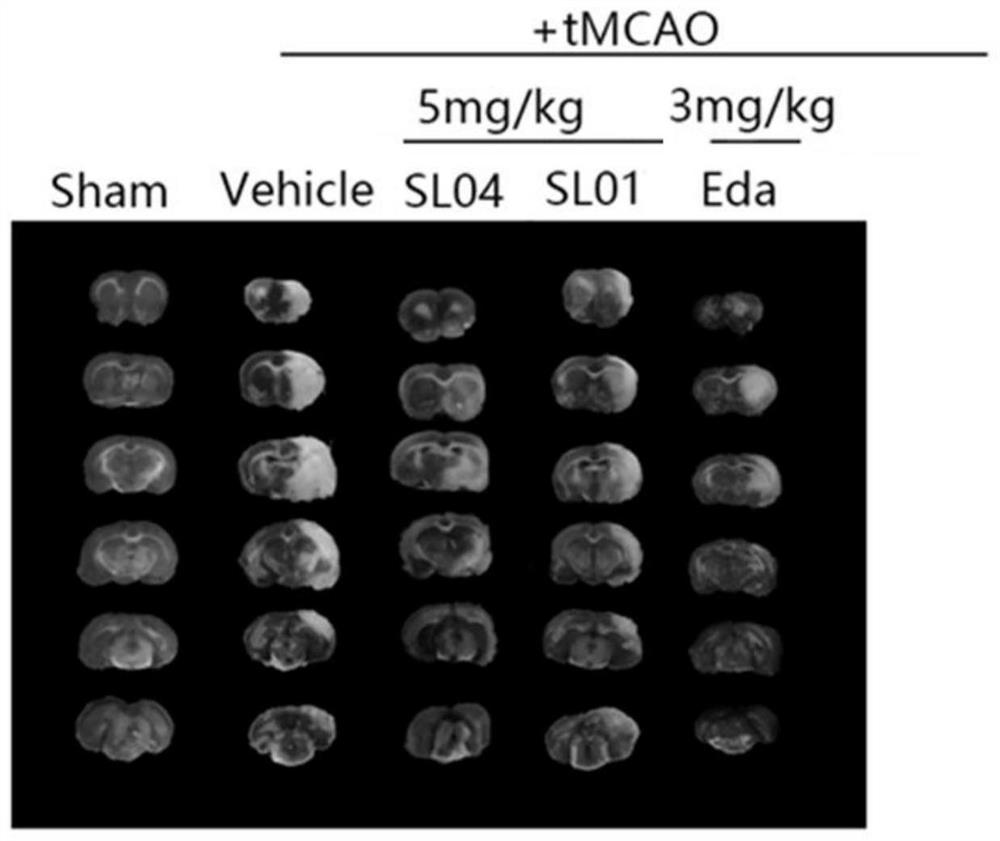
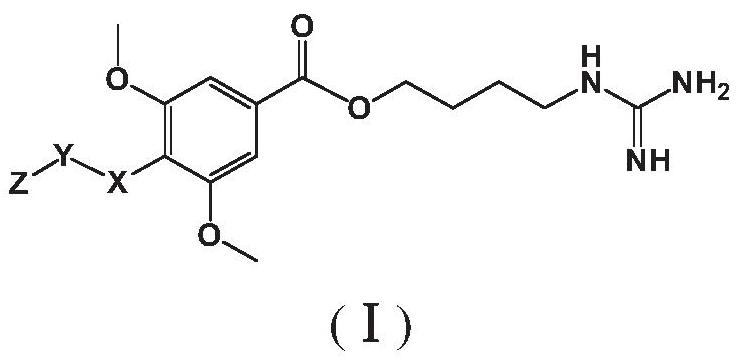
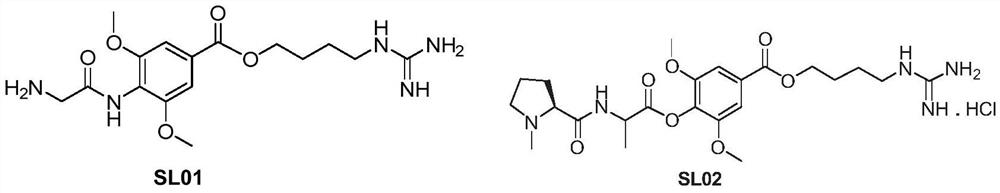
Leonurine derivative and application thereof in preparing medicine for preventing or treating ischemic cerebrovascular diseases
A cerebrovascular disease and leonurine technology, applied in the field of medicine, can solve the problems of poor fat solubility and low bioavailability of stachydrine, and achieve the effects of good treatment effect, repair of nerve cell damage and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Synthesis of 1,3-bis(tert-butoxycarbonyl)-2-methyl-2-isothiourea (1):
[0030]
[0031] At room temperature, S-methylisothiouronium sulfate (14g, 50.29mmol, 1eq) was dissolved in water / dichloromethane (75mL / 75mL) mixture, di-tert-butyl dicarbonate (14.2g, 63.37mmol , 1.3eq) was injected into the system, and then 75mL of sodium hydroxide solution with a concentration of 1mol / mL was added, and the reaction was stirred for 12h. Filter, beat with water at 50°C for 1.5h, filter, and dry the filter cake to obtain a white solid, namely compound 1 (13.4g, yield 92%); mp=115.4-116.2°C; 1 H NMR (500MHz, DMSO-d 6 ): δ 11.05 (s, 1H), 2.27 (s, 3H), 1.43 (d, J=7.7Hz, 18H). ESI-MS: m / zcalcd for C 12 h 22 N 2 o 4 S[M+H] + 291.14.Found: 291.13.
Embodiment 2
[0032] Embodiment 2: the synthesis of N, N'-Boc-N "-(4-hydroxyl-butyl)-guanidine (2):
[0033]
[0034] The obtained compound 1 (1.45g, 5mmoL, 1eq) was dissolved in DMF (10mL), and 4-amino-1-butanol (450mg, 5mmoL, 1eq) was added at 25°C, reacted for 9h, washed with water, and extracted with DCM , collected the lower layer solution, evaporated to dryness, column chromatography (petroleum ether: ethyl acetate = 2: 1), to obtain an amorphous white solid, namely compound 2 (1.48g, yield rate 90%); mp = 119.8-121.4 ° C ; 1 H NMR (500MHz, DMSO-d 6 ): δ 11.49(s, 1H), 8.28(t, J=5.0Hz, 1H), 4.41(s, 1H), 3.40(d, J=5.8Hz, 2H), 3.28(d, J=6.3Hz, 2H), 1.52(dd, J=14.4, 7.2Hz, 2H), 1.47(s, 9H), 1.45-1.4l(m, 2H), 1.39(s, 9H).ESI-MS: m / z calcd for C 15 h 29 N 3 o 5 [M+Na] + 354.20.Found: 354.01.
Embodiment 3
[0035] Embodiment 3: the synthesis of acetosyringic acid (3):
[0036]
[0037] Dissolve syringic acid (1g, 5.04mmol) in 7mL of acetic anhydride, heat to reflux for 2h, the temperature of the system drops to 50°C, add the reaction solution to 20mL of ice water, stir at 0°C for 1h, a white precipitate is observed Formed, filtered to obtain a filter cake, rinsed with an appropriate amount of ice water, and dried to obtain a white powder, that is, compound 3 (1 g, yield 83%); mp = 182.0-186.7 ° C; 1 H NMR (500MHz, DMSO-d 6 ): δ 7.28(s, 2H), 3.81(s, 6H), 2.27(s, 3H). 13 C NMR (126MHz, DMSO-d 6 ): δ167.76, 166.67, 151.64 (2C), 131.50, 129.25, 105.87 (2C), 56.11 (2C), 20.14. ESI-MS: m / zcalcd for C 11 h 12 o 6 [M+Na] + 263.05. Found: 262.79.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com