Application of leonurine to preparation of medicament for treating 2-type diabetes
A technology for type 2 diabetes and leonurine, which is used in drug combinations, antipyretics, metabolic diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
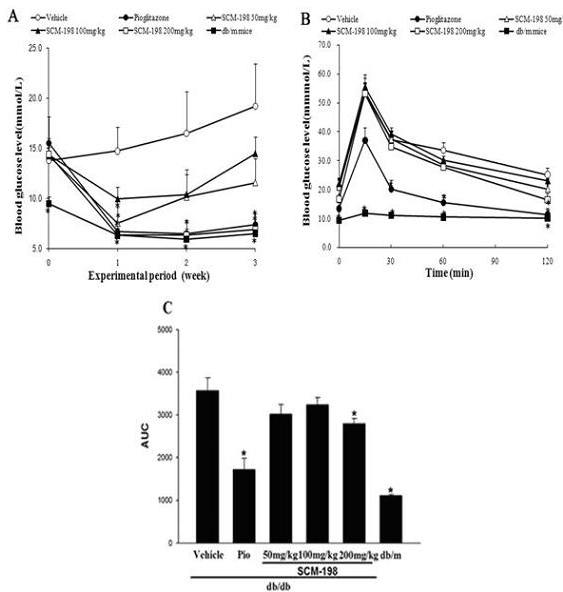
[0018] Example 1 Blood glucose monitoring, oral glucose tolerance test
[0019] (1) Experimental animals
[0020] 42 male C57BLKS / J db / db mice and 5 C57BLKS / J db / m mice (db / m mice were used as normal controls) were kept in their SPF grade animal room. The light alternated between light and dark every 12 hours, and they had free access to food and water. During the feeding and experiment process, the relevant guidelines for the management and protection of experimental animals were followed. The experimental animals used in this experiment were purchased from Shanghai Ruizhi Chemical Research Co., Ltd. The db / db mouse is a typical animal model of spontaneous type 2 diabetes. Its pathogenesis is caused by mutations in leptin receptors, and it is autosomal recessive. It has the characteristics of metabolic disorders similar to human type 2 diabetes: hyperglycemia, hyperinsulinemia, polyuria, diabetes and obesity.
[0021] (2) Experimental grouping and processing
[002...
Embodiment 2
[0027] Example 2 Detection of plasma indicators
[0028] Experimental animals and experimental grouping and treatment are the same as in Example 1.
[0029] Blood samples were collected in test tubes containing heparin, and centrifuged at 3000g for 15 minutes at 4°C; plasma insulin content was detected by ELISA; plasma triglyceride, total cholesterol, high-density lipoprotein and low-density lipoprotein were detected by Hitachi 7080 automatic biochemical analyzer Density lipoprotein and liver function indicators aspartate aminotransferase and alanine aminotransferase.
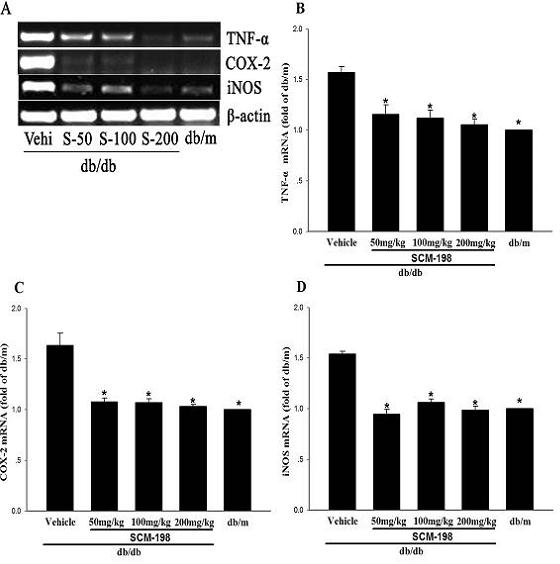
[0030] The results of plasma index detection showed that the plasma insulin content of the motherwortine (SCM-198) 100mg / kg, 200mg / kg treatment group was significantly improved compared with the db / db vehicle group; the motherwortine (SCM-198) 200mg / kg treatment Compared with the db / db vehicle group, the triglycerides in the group were significantly lower, while the high-density lipoprotein was significantly...
Embodiment 3
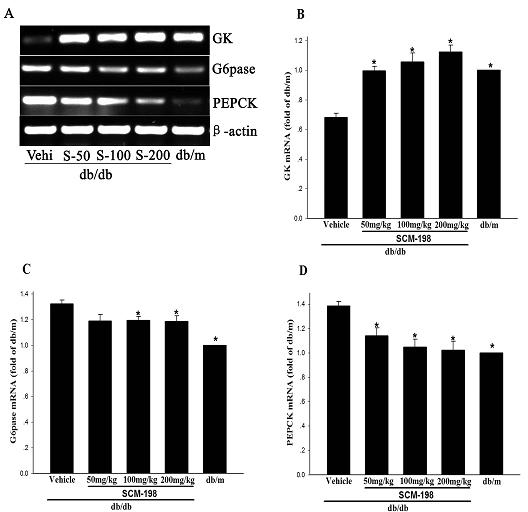
[0034] Example 3 Leonurine ( SCM-198) Effect on the expression of glucokinase (GK) gene, glucose-6-phosphatase (G6pase) and phosphoenolpyruvate carboxylase (PEPCK) gene
[0035] Experimental animals and experimental grouping and treatment are the same as in Example 1.
[0036] Glucokinase (GK) is the first rate-limiting enzyme in the glycolysis process, and the increase in its expression will promote the utilization of glucose and play a role in lowering blood sugar. Glucose-6-phosphatase (G6pase) and phosphoenolpyruvate carboxylase (PEPCK) are two key rate-limiting enzymes in the process of gluconeogenesis, and their increased expression will increase the process of gluconeogenesis and the production of glycogen. break down, and the result is an increase in blood sugar. In this example, the effect of motherwortine (SCM-198) on the expression of glucokinase (GK) gene, glucose-6-phosphatase (G6pase) and phosphoenolpyruvate carboxylase (PEPCK) gene was detected according t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com