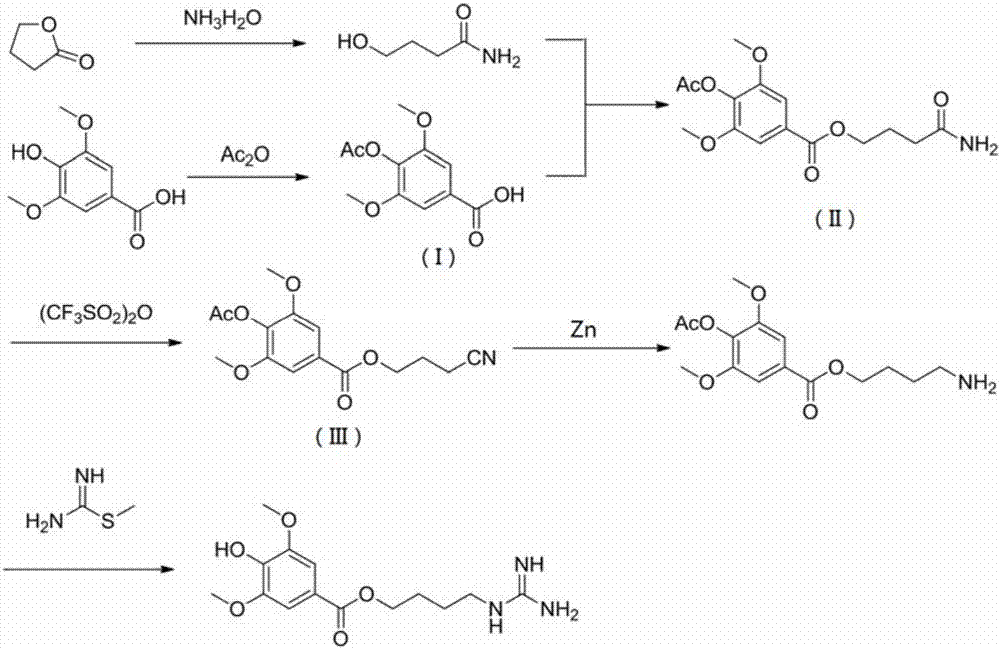
Synthetic method for leonurine
A technology of motherwort and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc., can solve the problems that starting materials are not easy to obtain, difficult to produce on a large scale, and high requirements for reaction conditions, Achieving excellent synthetic route, suitable for large-scale production, and more controllable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
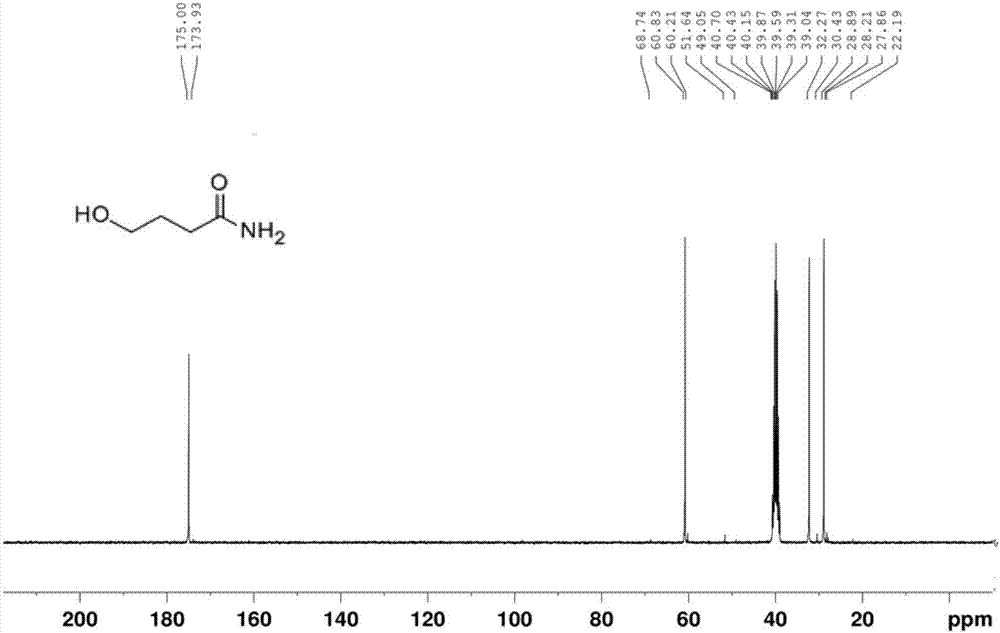
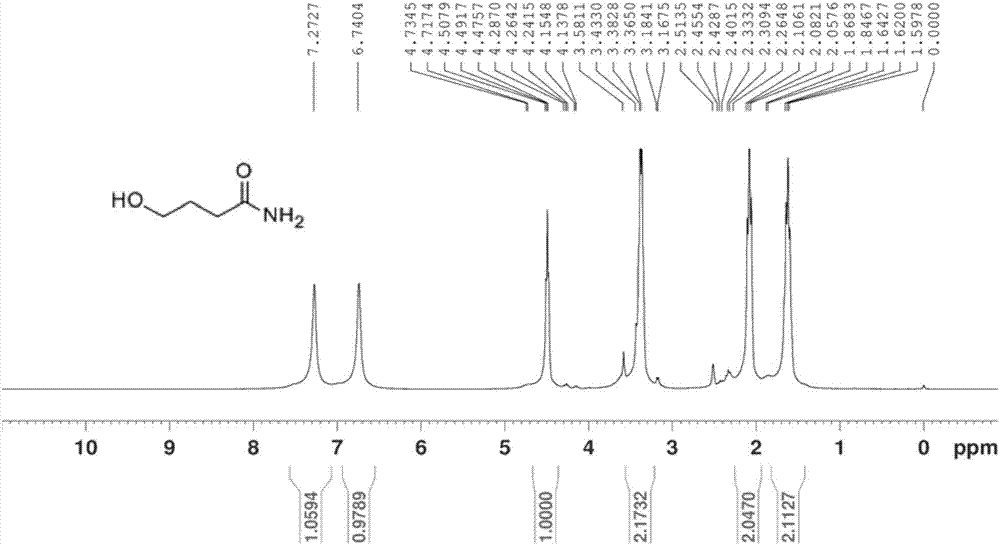
[0056] Embodiment 1: the synthesis of gamma-hydroxybutyric acid amide
[0057] Disperse 250 g (3.0 mol) of γ-butyrolactone in 1000 mL of ammonia water (28%), stir overnight, monitor the reaction by TLC, filter to obtain a pure white solid, figure 2 and image 3 Be respectively the carbon nuclear magnetic resonance spectrum and the hydrogen nuclear magnetic resonance spectrum of the pure white solid obtained, by figure 2 and image 3 The pure white solid product was identified as gamma-hydroxybutyric acid amide. The theoretical yield should be 250g, but the actual yield is 230g, the yield is 92%.
Embodiment 2
[0058] Embodiment 2: the synthesis of acetyl syringic acid (I)
[0059] Disperse 198g (1.0mol) of syringic acid in 1000mL of dichloromethane, add 97.6g (0.8mol) of 4-dimethylaminopyridine and stir evenly, slowly add 122.5g (1.2mol) of acetic anhydride dropwise at room temperature and stir for 20 hours, After spin-drying dichloromethane, after adding 900ml dehydrated ethanol thermally dissolving, add 600ml water again and stir evenly static crystallization, suction filtration, filter cake is washed once with 200ml water, oven dry obtains the compound (I) of 208g light brown crystal altogether, The theoretical yield should be 240g, and the yield is 86.6%.
Embodiment 3
[0060] Embodiment 3: the synthesis of acetylated syringyl ester (II)
[0061] Take 0.2mol (48g) of acetylsyringic acid (I), add 500ml of dichloromethane, successively add 0.25mol (47.75g) EDCI, 0.25mol (34g) HOBT and 0.25mol (34g) DIPEA, stir at room temperature for 1 hour, slowly add γ -Hydroxybutyric acid amide 0.25mol (26g), stirred at room temperature for 6h, added 200ml of water to quench the reaction, extracted, washed with saturated brine, and concentrated under reduced pressure to obtain 57g of compound (II) as a pale yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com