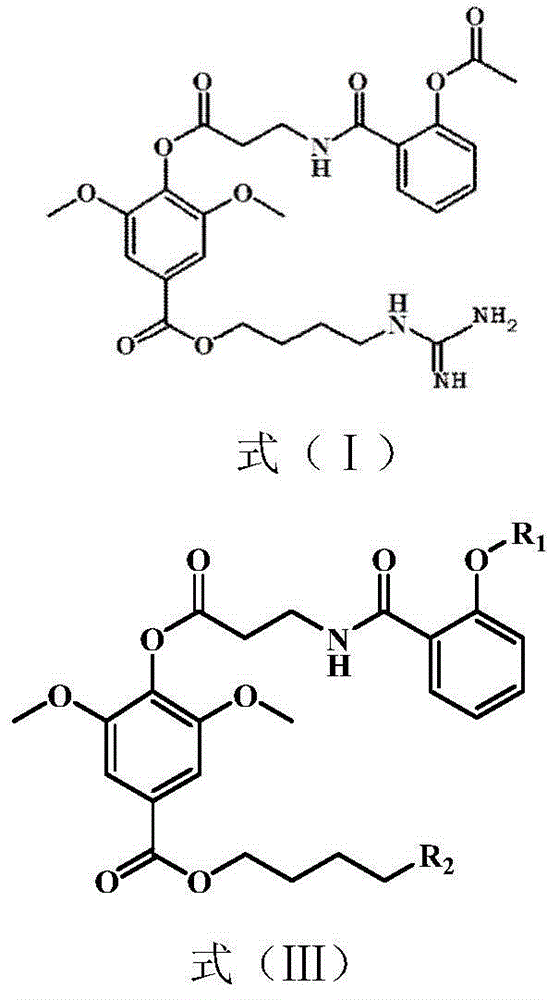
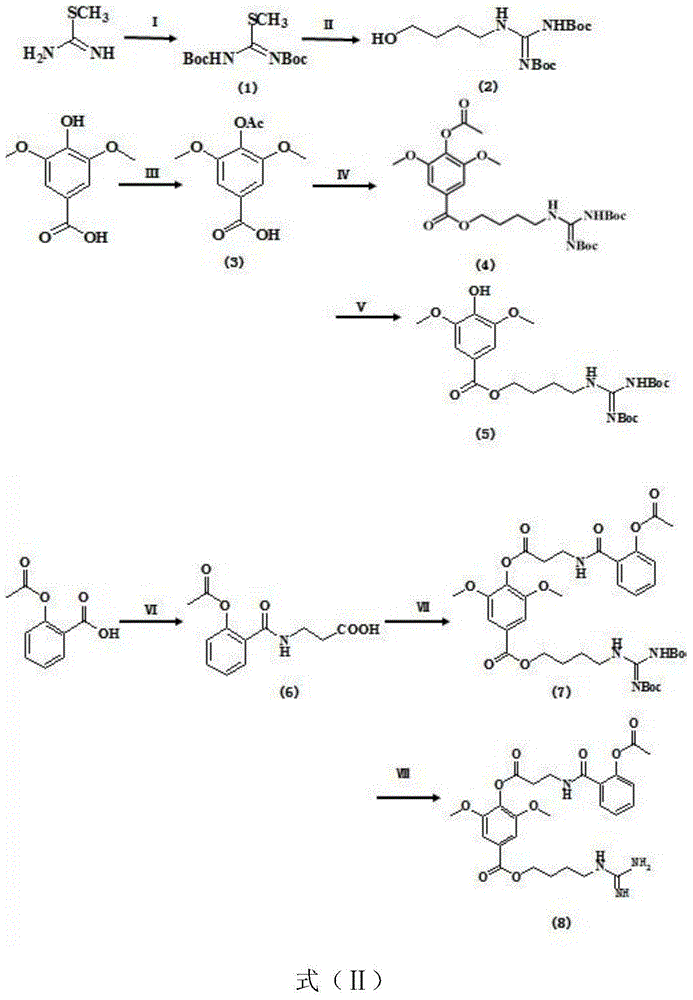
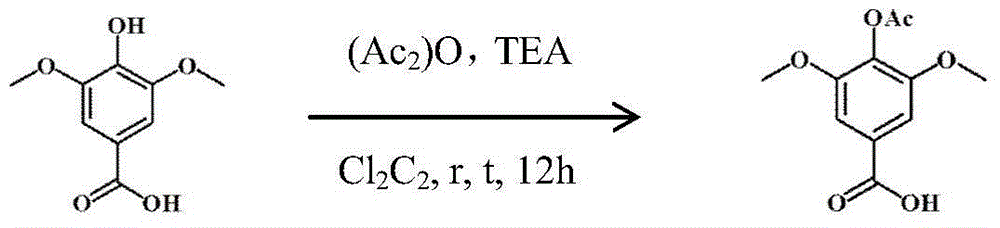Preparation method of leonurine and aspirin conjugate
A technology of leonurine and aspirin, which is applied in the field of preparation of leonurine and its analogs and aspirin conjugates, can solve the problems of low yield of esterification reaction, difficulty in operation, difficulty in purification and the like, and saves raw materials and shortens the reaction. effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis (3) of embodiment 1 acetylated syringic acid
[0035]
[0036] Using acetic anhydride (Ac 2 )O as an acylating agent, controlling the reaction time can make the productive rate reach more than 90%; the specific operation is: 100ml three-necked flask, add N2 protection, syringic acid (19.8g, 0.1mol) is added in 100ml dichloromethane, slowly drop Add 30ml (0.2mol) of triethylamine, slowly add 15ml (0.15mol) of acetic anhydride dropwise after all the samples are dissolved, monitor the progress of the reaction by TLC, after 12 hours of reaction, concentrate the reaction solution to 30ml by vacuum filtration and rotary evaporation, dropwise add 1N concentrated hydrochloric acid until the pH was 5, a large amount of white crystals were sucked out at this time, and the crude product of acetosyringic acid (22.7 g, 95%) was obtained by suction filtration and drying;
[0037] [In this example, 1N hydrochloric acid can be used to adjust the pH to 3, and then use ...
Embodiment 2B
[0038] The synthesis (1) of embodiment 2 Boc protection guanidino groups
[0039]
[0040] Dissolve S-methylisothiouronium sulfate (6.95g, 0.025mol) in 50ml of water, add 13.8ml (0.1mol) of triethylamine, after the sample is completely dissolved, to obtain solvent I, take (Boc)2O(21.8 g, 0.1mol) was dissolved in 10ml tetrahydrofuran, slowly added dropwise to solvent I, and 0.05g DMAP was added to the mixed solvent, reacted for 96h, and TLC monitored the reaction progress. Place DCM and water in a separatory funnel and extract 3 times, collect the DCM layer and concentrate to obtain a crude product, purify by silica gel column chromatography, and elute with petroleum ether: ethyl acetate (40:1) to obtain Boc-protected S-methyl isosulfide Urea (6.5 g, 91%).
Embodiment 34
[0041] The synthesis of embodiment 34-guanidinobutanol (2)
[0042]
[0043] Dissolve Boc-protected S-methylisothiourea (14.5 g, 0.05 mol) in anhydrous DMF, stir at room temperature for 1 h, and place the reaction vessel in a cold well (-5°C) after it is completely dissolved. Add 4-aminobutanol (6.75g, 0.075mo) at the bottom, take it out after 10min and react at room temperature for 3h, monitor the progress of the reaction by TLC, after the raw material point of S-methylisothiourea disappears, spin the reaction solution and place it in a separatory funnel DCM and water were extracted three times, and the concentrated DCM layer was collected and purified by silica gel column chromatography, eluting with petroleum ether: ethyl acetate (1:1) to obtain 4-guanidinobutanol (11.77 g, 71%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com