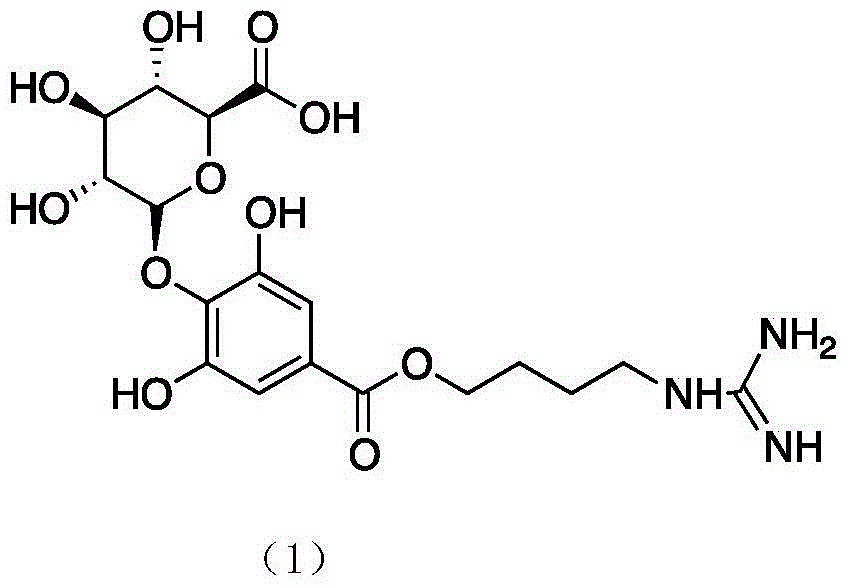
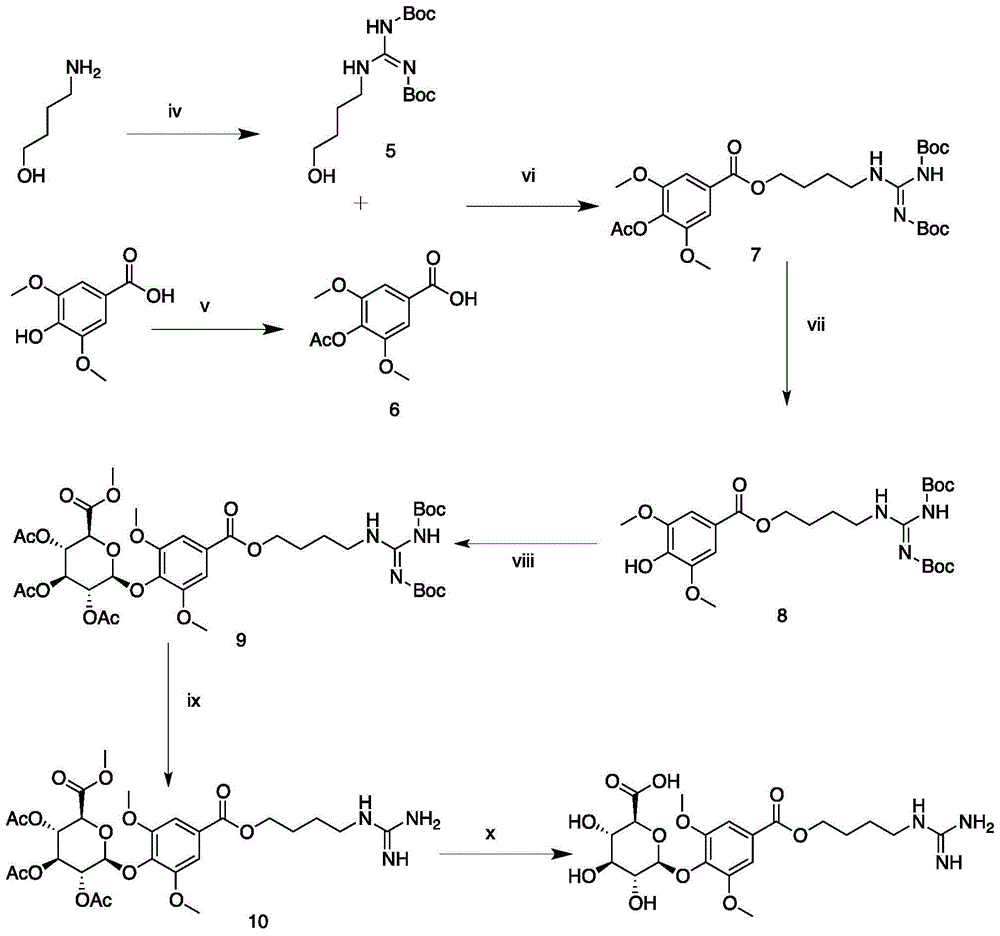
Leonurine metabolite and preparation method thereof
A technology of leonurine and metabolites, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of the preparation method and activity of the main metabolites of leonurine that have not yet been seen, and achieve good pharmacological activity prospects, Easy to control, cheap and readily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
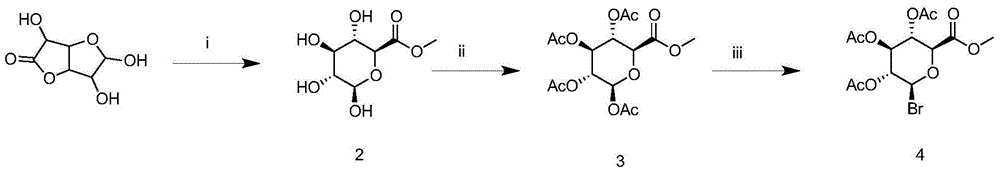
[0033] Synthesis of (2S,3S,4S,5R,6R)-1-methyl-3,4,5,6-tetra-hydroxy-tetrahydro-2H-pyran-2-methyl ester
[0034]
[0035] Add 100 mL of methanol and 0.4 g (0.01 mmol) of sodium hydroxide to a 250 mL flask, stir to dissolve, add 17.6 g (0.1 mmol) of glucuronic acid lactone, stir at room temperature, and add a small amount of sodium hydroxide during this period to keep the reaction solution. pH>8, a total of about 0.6 g of sodium hydroxide was added. After the raw materials were completely dissolved, stirring was continued for 1 h. The reaction solution was yellow and transparent. All methanol was evaporated under reduced pressure to obtain 16.8 g of a yellow syrupy viscous substance. The yield was 16.8 g. 100%.
Embodiment 2
[0037] Synthesis of (2S,3R,4S,5S,6S)-6-(methoxyformyl)tetrahydro-2H-pyran-2,3,4,5-tetra-acetyl-tetra-ethyl ester
[0038]
[0039] In a 250mL flask, add 60mL (0.6mol) acetic anhydride, 11g (0.05mol) compound 2, after dissolving, add dropwise a mixture of perchloric acid and acetic anhydride (0.3mL perchloric acid, 10mL acetic anhydride), control the drop Accelerate so that the temperature of the reaction solution does not exceed 40°C. After the dropwise addition, the reaction is stirred overnight, PE:EA=3:1 system, and separated by silica gel column chromatography to obtain 14.1 g of white solid with a yield of 86%.
Embodiment 3
[0040] Example 3 Synthesis of Compound 4
[0041]
[0042] 15g(0.04mol)(2S,3R,4S,5S,6S)-6-(methoxyformyl)tetrahydro-2H-pyran-2,3,4,5-tetra-acetyl-tetra-ethyl ester Dissolve in 33% HBr acetic acid solution (20 mL) under nitrogen protection, stir at 0 °C for 4 h, add 100 mL of dichloromethane, wash 5 times with ice water and saturated sodium bicarbonate respectively, combine the organic layers, add anhydrous sulfuric acid Dry over sodium and evaporate the solvent to give (2S,3R,4S,5S,6S)-2-bromo-6-(methoxyformyl)tetrahydro-2H-pyran-3,4,5-tri-acetyl- Triethyl ester (compound 3), yield 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com